How to Cite | Publication History | PlumX Article Matrix
Amal A. Al Mousa1* , Fatimah S. Al-Khattaf1
, Fatimah S. Al-Khattaf1 , Ashraf A. Hatamleh1
, Ashraf A. Hatamleh1 , Jana A. Aljurays2
, Jana A. Aljurays2 , Hadeel S. alabdulhad2, Nadine M. S. Moubayed1
, Hadeel S. alabdulhad2, Nadine M. S. Moubayed1 and Raneem S. Aldouhan1
and Raneem S. Aldouhan1
1Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia.
2King Saud University, Vice Rectorate for Graduate Studies and Scientific Research, Deanship of Scientific Research, Research Assistant Internship Program, Riyadh, Saudi Arabia.
Corresponding Author E-mail:aalmosa@ksu.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/3174
ABSTRACT: The unregulated utilization and extensive disposal of synthetic polymers, resulting in excessive buildup in natural ecosystems, has become a significant cause for alarm. Consequently, there is a pressing need for the development of sustainable plastic degradation methodologies. In the present study, the potential of new Bacillus strains isolated from five petroleum stations to degrade plastics LDPE (Low-density polyethylene) and polyester: plastic bags, bottles and cups was investigated. Following bacterial screening and molecular identification, two strains with no previously known plastic removal ability, Bacillus cytotoxicus SB 9 and Bacillus sp. revealed good plastic biodegradation ability at 37 ºC surfaces with Scanning Electron Microscopy (SEM) and pronounced weight loss were observed with the mixture of bacterial isolates mainly on the plastic cup, bottle and then the bag. These results indicate the ability of these novel Bacillus sp. to develop a synthetic polymer degrading mechanism as a promising, smart eco-friendly plastic waste management for the soil environment.
KEYWORDS: Bacillus sp, Polymers; Plastic waste management; Sustainability; Smart biodegradation
Download this article as:| Copy the following to cite this article: Mousa A. A. A, Al-Khattaf F. S, Hatamleh A. A, Aljurays J. A, Alabdulhad H. S, Moubayed N. M. S, Aldouhan R. S. Enhancing the Biodegradability of LDPE Plastic Waste through Sustainable Bacterial Isolates from Saudi Arabian Soils. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Mousa A. A. A, Al-Khattaf F. S, Hatamleh A. A, Aljurays J. A, Alabdulhad H. S, Moubayed N. M. S, Aldouhan R. S. Enhancing the Biodegradability of LDPE Plastic Waste through Sustainable Bacterial Isolates from Saudi Arabian Soils. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/3RzEymt |
Introduction
Plastic accumulation in the environment is one of the major problems in the world which has increased lately with the Covid- 19 pandemic due to the over use of PPE kits, disposable gloves and masks. Plastic are synthetic polymers made up of high molecular weight (petrochemicals) 1. a long chain of hydrocarbons, such as polyethylene and polystyrene, are used extensively in daily life as packaging material and other industrial and agricultural applications 2-3.Plastic waste can persist in the environment for centuries. The same properties that make plastics so useful — their durability and resistance to degradation — also make them nearly impossible for nature to completely break down. In Saudi Arabia (KSA), there is a substantial annual generation of municipal waste, amounting to 15.3 million tons per year, with plastic constituting 17.4% of this waste).4. Plastic waste ranks as the second most prevalent waste category in KSA. This situation is mainly attributed to the extensive use of disposable items, particularly in the holy cities of Makkah and Madina, where thousands of pilgrims visit annually. The disposal of plastic waste in KSA primarily occurs within municipal solid waste landfills. Traditional recycling methods only recycle a fraction of the plastic waste, prompting the exploration of waste treatment techniques such as anaerobic digestion and pyrolysis processes. As demonstrated by Miandad et al. (2016), 1. pyrolysis is an effective means of converting plastic waste into liquid fuel. Recently, there has been a growing focus on utilizing anaerobic digestion technology for solid organic waste treatment. Anaerobic digestion is defined as “the microbial degradation and stabilization of organic materials under oxygen-free conditions, which leads to the production of stable biomass and biogas”. 5. However, despite the efficiency of these waste treatment approaches, plastic is at risk of reaching the marine environment. Over recent years, there has been a sharp increase in the influx of plastic pollution into aquatic ecosystems, and this is projected to more than double by 2030. Rivers frequently serve as conduits for transporting plastic waste from inland areas to the sea, contributing significantly to ocean pollution. An astonishing 8 million tons of plastic enter the world’s oceans annually 6. It is important to note that most plastic items never truly vanish; instead, they fragment into smaller particles. Farm animals or fish frequently ingest these microplastics, mistakenly identifying them as food. Furthermore, these microplastics have been detected in most of the world’s tap water, posing a threat to human and animal health. This situation has dire consequences for human health, the global economy, biodiversity, and the climate. The COP26 climate change conference highlighted the role of plastics as a climate-related issue. Life cycle analyses reveal that marine litter and plastic pollution significantly impact the global environment (UNEP). The KSA has contributed several national and international procedures to reduce the effects of waste at both national and global levels during the G20 summit.
Microbial plastic waste biodegradation has become more important because of its low cost and eco-friendly plastic removal ability, primarily because all chemical and physical removal methods previously used for these pollutants release hazardous metabolites or by- products, leading to environmental problems. Several types of soil habitat microorganisms, including bacteria and fungi, play a major role during different steps in the degradation of plastics. These microbes, together with their enzymes, break down complex polymers into smaller bio-molecules, such as oligomers, dimers, and monomers. These can pass the semipermeable outer membranes of the microbes for use as carbon and energy sources. Microorganisms can first break down the polymers inside them, and then disseminate their enzymes to discharge extracellular proteins which cleave monomer chains, making them more easily used by microorganisms 7. Microbial biodegradation is mainly related to the polymers’ crystallinity and atomic weight 8-9.which affects the rate of substantial plastic removal. Many previous studies demonstrated the ability of microorganisms to remove a single kind of plastic9. Thus a comprehensive study into biodegradation of all main kinds of plastic is necessary. For this, the present study aimed to 1) isolate and identify bacterial strains in soil samples of five different local petroleum stations; 2) screen and evaluate the potential ability of these bacterial isolates to degrade two main types of plastic LDPE and polyester present together in the cultivation media, and 3) evaluate the bacterial plastic biodegradation ability through both Scanning Electron Microscopy (SEM) and plastic weight loss determination as future alternative polymer degraders.
Materials and Methods
Sample collection
Soil samples, were collected, in sterile glass bottles, from five different petrol stations in Riyadh city, Saudi Arabia. 70-100g of soil samples, at 5-15cm depth, were taken from each station. Samples were kept cold and immediately transferred to the laboratory for analysis.
Sampling was made at the same day, under the same environmental condition in September, 2021; both physical parameters soil temperatures and humidity were recorded as 37℃ and 20%respectively.
Two main types of plastic, LDPE (low density polyethylene) and polyester, in plastic bags,cups and bottles, were used in this study. These plastic materials were purchased from a local market in Riyadh and were pretreated before use in the experiment.
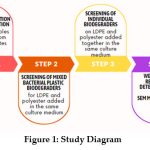 |
Figure 1: Study Diagram Click here to view Figure |
In-situ Bio-Degradation of plastics
Screening, Isolation and identification of sustainable bacterial strains.
1gm of each of the five collected soil samples was serially diluted using conventional serial dilution method. 0.1mL of dilution 10-8 was plated on nutrient agar plates (NA) in triplicates. All plates were incubated for 1-day at 37°C. Five single colonies of mostly occurring isolates, were selected and purified on nutrient agar plates. These five potential plastic degraders were initially distinguished based on their physical characteristics and Gram staining. Subsequently, a more precise identification was achieved through molecular techniques.
To ascertain the identity of these pure isolates, the 16srRNA gene sequence was employed. DNA extraction was carried out following the guidelines in the pure link mini prep genome kit (Invitrogen, USA). Subsequently, the extracted DNA’s concentration and quality were assessed using the Genova nanodrop (Italy). PCR was employed to amplify the target DNA segment, using the universal primer set 1492R (5’-AAGGAGGTGATCCAGCCGCA-3’) and 27F (5’-AGAGTTTGATCCTGGCTCAG-3’). The PCR reaction was conducted in a total volume of 25 μL within a Genepro thermal cycler (Bioer, China). The reaction mixture included GoTaq Gareen Master Mix (Promega, USA), 10 μmol of both forward and reverse primers, and 2 μL of the DNA sample. The PCR cycling conditions were as follows: an initial denaturation step at 94˚C for 2 minutes, proceeded by 35 cycles consisting of denaturation at 94˚C for 15 seconds, the annealing of each primer at 63˚C for 1 minute and 72˚C for 2 minutes, and a final elongation step at 72˚C for 5 minutes, following the protocol described by Moubayed et al. (2019). The amplified products were observed through electrophoresis on a 1.5% agarose gel. In this analysis, samples were compared to Bacillus subtilis ATCC 6633, serving as a positive control strain, with all bands consistently observed at approximately 1500 bp 11. Following identification, bacterial isolates were then screened for their plastic biodegradation ability first on solid plates containing each of the plastic strips. Those presumptive bacterial isolates, showing good inhibition zone indicating an efficient biodegradation ability, were selected for further analysis.
DNA sequencing
Following the PCR, the resultant PCR products underwent purification utilizing the Qiagen QIA 250 Qiagen purification kit (Germany). Subsequently, these purified products were sequenced using the Applied biosystem sequence analyzer (Spain). For the sequencing procedure, the ABI PRISM® BigDye™ Terminator Cycle Sequencing Kit (version 3.1) was employed following the manufacturer’s instructions. The same primer set, namely 1492R and 27F, was used for this sequencing process. To ascertain the identity of the DNA sequences and explore their evolutionary relationships, The National Centre for Biotechnology Information (NCBI-BLAST) software was utilized. This analysis allowed the comparison of the 16SrRNA genetic marker sequences from the standard positive control strain with those of the studied isolates.
Construction of the Phylogenetic Tree
To elucidate the evolutionary relationships of the studied isolate, a phylogenetic analysis was conducted employing the neighbor-joining method, implemented through the Mega X .12. Specifically, the 16srRNA gene sequence of the candidate strain was juxtaposed with sequences available in the National Center for Biotechnology Information (NCBI) GeneBank, facilitated by the BLAST (Basic Local Alignment Search Tool).
Pre-treatment of polythene
The plastic bags, bottles and cups used in this study were cut into small strips of 1*1 cm and transferred to a fresh solution of Tween 80, bleach, and distilled water (70:80:983 mL), respectively, and stirred for 30–60 mins. Following this, the strips were washed by stirring with distilled water for 1 hour. Finally, the strips were aseptically placed in 70% ethanol for 30 min ready to be inoculated in the culture media for microbial plastic biodegradation determination.
Microbial Degradation of Polythene and Plastic under laboratory Condition
Sterilized plastic strips were placed altogether in 50 ml nutrient broth culture medium (NB) (Oxoid, USA) and were then inoculated with (0.5ml) overnight cultures of each of the bacterial isolates having the highest plastic removal ability. In parallel, Other flasks were similarly inoculated with 3 plastic strips and a mixture of these two best plastic bio- degraders as a comparison between single or consortium plastic bio-degraders ability. Control was maintained with plastic strips in a microbe-free medium. Inoculated flasks were kept in a shaking incubator (180rpm) for 7days-10 days at 37˚C. Following the incubation period, plastic strips were collected, washed thoroughly using distilled water,shade-dried and then weighed for weight reduction calculation using the following formula13. The experiment was repeated three times and average weight was recorded.
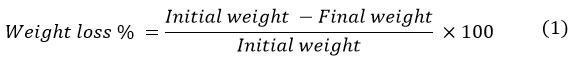
Plastic strips surface modification analysis using Scanning Electron Microscopy
The LDPE and polyester plastic strips, both in their original state and after undergoing degradation, were subject to surface examination using Scanning Electron Microscopy (SEM) with a JEOL Model JSM–6390LV instrument. To prepare the samples for SEM analysis, they were affixed to 1.2 cm aluminum disks using double-sided black carbon tape. Subsequently, a thin layer of gold was uniformly applied to the samples within a vacuum chamber utilizing argon gas and an electric current of approximately 3 mA. These samples were then visualized at high magnification 14. SEM films were processed at the central laboratory,King Saud University.
Results
Bacterial screening activity
Five dominant isolates were obtained from petroleum station soil samples. All five bacterial isolates were primarily screened for their plastic removal ability on NA plates containing strips of each form of plastic included in the study (Data not shown). Only 2 presumptive isolates showed distinct clear zone on agar plates and significant reduced plastic weight loss and were selected for further analysis (Fig. 2). These two potent plastic bio-degraders exhibited a very similar pattern of degrading ability when inoculated separately or together on an NB culture medium containing samples of the three types of plastic. The maximum degrading activity was observed on the plastic cups, followed by the bottles; the lowest activity was observed on the plastic bags. Biodegradation was more pronounced when the bacterial strains were combined.
 |
Figure 2: Primary screening for B1 and B2 plastic degradation ability in the culture medium for 7 days at 37c. (a) reveals almost complete degradation of the bacterial isolate B1 for the 3 types of plastic strips in study added altogether in the same culture medium. Click here to view Figure |
Evaluation of the LDPE and Polyester-Degrading Microorganisms Through Weight Loss Analysis
Determining weight reduction is critical for studying polymeric biodegradation intended to reduce solid waste generation in the environment. Following incubation (7–10 days) with continuous shaking, the maximum weight reduction was observed and measured. It was noted that bacterial mixture of both bacterial strains (B1+B2) Bacillus cytotoxicus OP566858 strain (B1) and Bacillus sp. (B2), greatly degraded the plastic cup strip, with less degradation observed on the bottle strip, whereas the plastic bag remained almost intact. Similarly, Bacillus cytotoxicus OP566858 strain (B1) and Bacillus sp. OP566859 (B2) each showed equal degrading ability when applied separately. The best degrading activity was observed on the plastic cup, followed by the plastic bottle, and a negligible effect was observed on the plastic bag. The combined activity of both strains was more pronounced compared to the ability of each bacterial isolate on its own (see Table 1).
Table 1: weight loss reduction of LDPE and polyester samples.
| Bacterial isolates | Average weight loss reduction in grams | Control | Weight loss percentage (%) | ||||
| Bag | Cup | Bottle | Bag | Cup | Bottle | Bag Cup Bottle | |
| B1 | 0.0026 | 0.01 | 0.037 | 0.0027 | 0.01 | 0.039 | 4 0 5 |
| B2 | 0.0027 | 0.01 | 0.038 | 4 0 3 | |||
| Mixture | 0.0027 | 0.009 | 0.036 | 4 10 8 | |||
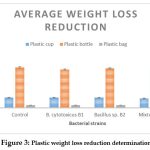 |
Figure 3: Plastic weight loss reduction determination. Click here to view Figure |
The highest biodegrading activity was observed with the bacterial mixture B1+B2 compared to the control and to the each of the strains B1 and B2. Plastic cups were observed to be highly degraded in the bacterial mixture than in the individual activity of B1 and B2. negligible or no biodegradation activity was observed on the plastic bag and bottle neither with single strains nor with the bacterial mixture. On the other hand, both strains showed similar ability to degrade both types of plastic LDPE and polyester revealed by the plastic cups, the plastic bottles and the plastic bag correspondingly.
Furthermore, percentage of plastic weight reduction of plastic materials used in this study was calculated using the following equation:

Highest weight loss reduction percentage (10%) was observed for the plastic cup followed by and 8% weight reduction for the bottle particularly with the bacterial mixture, whereas plastic bag showed only a 3% weight loss reduction with the bacterial consortium. Both strains separate showed lower weight loss reduction (3-5%). for all three types of plastic used compared to the bacterial consortium.
Identification of Bacterial Isolates
The bacterial isolates displaying superior biodegrading capabilities—denoted as B1 and B2—underwent a 16SrRNA partial analysis. This analysis involved a comparative examination against the NCBI GeneBank database, leading to the determination that both isolates belong to the Bacillus genus. Specifically, they were assigned the accession numbers B1 (Bacillus cytotoxicus) with the accession code OP566858 and B2 (Bacillus sp.) with the accession code OP566859. The construction of a neighbor-joining phylogenetic tree further clarified the placement of these isolates within the Bacillus genus. Notably, the tree indicated that these isolates fall within a branch that includes Bacillus cytotoxicus and Bacillus spp. (specifically, Bacillus paramycoides), as illustrated in Figures 4 and 5.
 |
Figure 4: Phylogenetic positioning of isolate B1 within the Bacillus genus using 16S rDNA. Click here to view Figur |
Figure 5. Phylogenetic placement of isolate B2 within the Bacillus genus utilizing 16s rDNA. The construction of this phylogenetic tree was accomplished using the neighbor-joining method through Mega X software. The objective was to discern the genetic relationships, as inferred from the 16S rDNA sequences, between isolate B2 and closely related type strains. Isolate B2 demonstrated an equivalent degree of relatedness to Bacillus thuringenesis SRG2 and Bacillus paramycoides FA85. The horizontal bar featured in the figure symbolizes the extent of evolutionary differentiation among these entities.
 |
Figure 5: Phylogenetic placement of isolate B2 within the Bacillus genus utilizing 16s rDNA. Click here to view Figure |
Surface modification confirmed by SEM
Degradation and morphological changes in polyester and LDPE strips after bacterial treatment were analyzed using SEM and observed as the formation of rough surfaces,cracks/holes/grooves, and the attachment of the bacterial cells to the surface of some plastic strips. SEM images, captured at magnification scales from 1500 to 4000, demonstrated distinct instances of localized surface degradation on plastic samples subjected to bacterial treatment. In contrast, the external surfaces of untreated plastics remained pure and exhibited a smooth texture.
 |
Figure 6: SEM micrographs for plastic bag. a) control showing the surface free of bacteria.b, c) showing B1 and B2 strains attached to the plastic bag surface with slight formation of grooves. Click here to view Figure |
Figure 7- SEM analysis for plastic bottle surface. a) control surface showing few cracks or grooves. b, c) B1 and B2 isolates attached on the bottle surface forming biofilm and increased grooves as an indication of plastic utilization and degradation. d) demonstrates the formation of deep holes similarly as seen on the plastic bag surface with the mixture of both strains in study indicating the bacterial consortium plastic degradation activity.
 |
Figure 7: SEM analysis for plastic bottle surface. a) control surface showing few cracks or grooves. Click here to view Figure |
Figure 8. Plastic cup surface SEM micrographs. a) control plastic cup surface. b and c) indicate B1 and B2 isolates growth and attachment on the cup surface with the formation of grooves and small holes, whereas, d) demonstrates the pronounced effect of the bacterial mixture as plastic bio-degraders of the cup surface by the formation of biofilm and increased grooves and deep holes.
 |
Figure 8. Plastic cup surface SEM micrographs. a) control plastic cup surface. b and c) indicate B1 and B2 isolates growth and attachment on the cup surface with the formation of grooves and small holes, Click here to view Figure |
Discussion
Due to the growing accumulation of plastic wastes, which causes critical environmental problems, it is urgent to develop new approaches for plastic waste removal. Some current attempts utilize microbes and their enzymes as alternatives to the mechanicochemical methods used previously. This study focused on using different types of plastic, namely LDPE and polyester, in three different materials from plastic cups, bottles, and bags, combined in the same culture media to be degraded by either a single strain or a mixture of new strains of Bacillus sp. isolated from soil samples collected from local petroleum stations. The evaluation of degradation efficiency was conducted through the measurement of weight loss observed in plastic strips submerged in the nutrient broth culture medium.The difference in weight reduction could be due to the difference of bacterial species and their metabolic pathways and or to the surface area of the plastic material itself. Larger surface area allows increased bacterial attachment and hence increased ability to degrade 15. Recently, a number of studies demonstrated the ability of various microorganisms and enzymes of degrading plastics, they have reported that Bacillus spp. are plastic degraders of only a specific type of plastic low density polyethylene LDPE 16-17-18 . however, in this study, a bacterial mixture of Bacillus sp. Was able to degrade different types of plastic in one nutrient culture medium at optimal conditions of 37c over 7-10 days; greater weight loss, and thus, greater biodegradation were found for LDPE than for polyester, this finding parallels that of Muhonja et al. 19. who reported that Bacillus cereus showed potent degrading activity on LDPE. In this study, both bacterial isolates, identified as Bacillus cytotoxicus OP566858 (B1) and Bacillus sp. OP566859 (B2), were noted to have a high synergistic affinity for attachment with the plastic materials studied. They degraded two different types of plastic: LPDE by 3-10% and polyester by 4% (Figure 3), A higher synergistic biodegrading ability was noted on the plastic cup samples (10%) and a lower ability on those from both the plastic bottle and bag (Table 1) and (Figure 3). This agrees with Dang et al.’s 20 findings, who reported that Bacillus sp. BCBT21 had trouble degrading plastic bags. Significant evidence of the microbial treatment, in the form of folding, erosion, and some scattered bacterial colonization, were observed on the surface of the LDPE and polyester films in SEM micrographs (Figures 6-8). More pronounced grooves and holes were revealed on the surface of the plastic cup with the bacterial mixture compared to the bottle and bag (Figure8), indicating as such an increase in the roughness of the film and subsequently, loss in mass is evidenced on the plastic surface. A similar pattern of biodegrading activity was also observed with both strains when they were inoculated separately in the culture medium. Both strains Bacillus cytotoxicus OP566858 (B1) and Bacillus sp. OP566859 (B2) showed surface modification mainly on the cup compared to the bottle surface and a negligible effect was observed on the plastic bag surface (Figures 6- 8). This plastic weight reduction (Figure 3) and (Table 1) is mainly related to the biological process of the bacterial isolates and not to the chemical associate present in the media, in agreement with Helen et al. 21-22. Successful plastic biodegradation requires utilization of all plastic organic components by bacteria 23 through different bond cleavage and enzymatic activities. This occurs in several steps, either aerobically or anaerobically—first by microbial attachment and colonization on the plastic surface, followed by extracellular enzyme(depolymerases) secretion, which can convert these polymers into low-molecular weight compounds or monomers 24-25-26. Some of these compounds may ultimately be mineralized as CO2 and water through bacterial metabolism or be exploited through metabolic pathways for valuable product biosynthesis27. Upon contact with the polymer surface, attached microbes initiate hydrophobic interactions and inevitably change the plastic’s properties. With the presence of inorganic ions and other molecules promoting attachment, bacteria will penetrate deep inside the polymer, secrete biosurfactants, and increase both hydrolysis and conductivity by water accumulation, leading to plastic discoloration 28.
In the current study, LDPE exhibited a significantly higher degradation rate (10%) when compared to polyester (4%). This disparity in biodegradation rates between LDPE and polyester can be attributed to the presence of distinct enzymes required for the degradation of different plastic types, and these enzymes may exist in varying concentrations 29. For instance, lacasse and alkane hydrolase enzymes have been identified as members of the AlkB enzyme family. Lacasse is frequently associated with the degradation of HDPE, while alkane hydrolase plays a pivotal role in LDPE degradation 30-31. Additionally, research findings have supported the involvement of laccase enzymes produced by Bacillus cereus in the degradation of low-density polyethylene, particularly after nine weeks of incubation 32. Another study shed light on the degradation process, indicating that the plastic surface is subject to attack by extracellular enzymes produced by Alcaligenes faecalis and Bacillus sp., including CMCase, protease, xylanase, and lipase 33. Thus, it is evident that enzymatic degradation plays the most important role in polymers degradation.
During bacterial growth, different metabolism rates with the help of different enzymes for an energy uptake from carbon-carbon backbone plastic cleavage occurred. This was observed as binding and colony-forming on the surface of the polymers and increased formation of holes and grooves on the surface, as suggested by Sowmya et al. 32. who also reported that SEM analyses are the best tool to confirm microplastic bacterial biodegradation (Figures 6-8).
An additional investigation corroborated the degradation process through the development of a biofilm on the plastic surface. In the context of LDPE degradation, SEM analysis demonstrated that Bacillus amyloliquefaciens displayed adherence and proliferation when LDPE was the only carbon source .16. Similarly, in a separate study focused on the deterioration of polystyrene, it was observed that cavities and holes on the material’s surface increased in size following the treatment 34. Similarly, both Bacillus cytotoxicus (B1) and Bacillus sp. (B2) strains showed a promising LDPE biodegrading ability either combined or separate. This was because LDPE has high hydrophobicity and an increased molecular weight of 30 kDa 35 and is thus hard to degrade. Conversely,little or no significant effect was observed on polyester.
This activity was revealed by the weight loss reduction and by the formation or increased depth of cavities on the surface of the plastic materials studied. Thus, structurally different plastic biodegradation and the end-products are species-enzyme dependent. Based on an understanding of both the depolymerase mechanism and the microbial metabolic pathway, it is interesting to build microbial cell factories capable of degrading polymer wastes and utilize their small products as chemicals with high value, establishing cyclic plastic consumption. Thus, a full understanding of the enzymatic biodegradation mechanism is needed for optimizing plastic biodegradation, leading to optimal bioengineering approaches.
Conclusion
In this study, Bacillus cytotoxicus (B1) and Bacillus sp. (B2), obtained from soil collected from five different local petroleum stations, either mixed or separated, were found to be the most promising strains for LDPE, and to a lesser extent, polyester bio-degraders. This removal ability was revealed by bacterial attachment and the formation of cracks confirmed by SEM analysis. Hence, this study screened and identified new bacterial strains with the ability to degrade different kinds of plastic in one culture medium, producing a significant outcome in terms of plastic degradation and sustainability, compared to the previously used methods. Additional tests, such as FTIR analysis, surface roughness, are needed, in the future work, to deeply elaborate and give a clear understanding about the microbial biodegradation.
Acknowledgments
The authors would like to thank the Distinguished Scientist Fellowship Program through researchers supporting project number (RSP2023R227), King Saud University, Riyadh, Saudi Arabia.
Conflict of Interest
We declare no conflict of interest.
Funding Sources
Distinguished Scientist Fellowship Program through researchers supporting project number (RSP2023R227) ), King Saud University, Riyadh, Saudi Arabia.
References
- Miandad, R.; Anjum, M.; Waqas, M.; Ahmad, I.; Omar, Z.; Alafif, A.; SirajAburiazaiza, A.; Abou, M.; Barakat, E-F.; Akhtar, T. Solid waste management in Saudi Arabia: A review. In Journal of Applied Agriculture and Biotechnol., 2016, 1,13-26. http://jaab.uaar.edu.pk
- Esmaeili, A.; Pourbabaee, A. A.; Alikhani, H. A.; Shabani, F.; Esmaeili, E. Biodegradation of Low-Density Polyethylene (LDPE) by Mixed Culture of Lysinibacillus xylanilyticus and Aspergillus niger in Soil. PLoS ONE, 2013, 8, e71720. https://doi.org/10.1371/journal.pone.0071720
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Science of the Total Environm., 2021, 759,143536. https://doi.org/10.1016/j.scitotenv.2020.143536
- Nizami, A. S.; Rehan, M.; Ouda, O. K.M.; Shahzad, K.; Sadef, Y.; Iqbal, T.; Ismail, I. M. I. An argument for developing waste-to-energy technologies in Saudi Arabia. Chemical Engineering Transactions, 2015, 45, 337–342. https://doi.org/10.3303/CET1545057
- Chen, Y.; Cheng, J. J.; Creamer, K. S. Inhibition of anaerobic digestion process: A review. Bioresource Technol., 2008, 99, 4044–4064. https://doi.org/10.1016/j.biortech.2007.01.057
- https://www.independent.co.uk/climate-change/news/plastic-waste-in-ocean-to-increase-tenfold-by-2020-10042613.html
- Bhardwaj, H.; Gupta, R.; Tiwari, A. Communities of microbial enzymes associated with biodegradation of plastics. J. Polymers Environm., 2013, 21, 575-579. doi: 10.1007/s10924-012-0456-z
- Singh, G.; Singh, A. K.; Bhatt, K. Biodegradation of polythenes by bacteria isolated from soil. Intern. J. Research Develop. Pharmacy & Life Sciences, 2016, 5, 2056-2062
- Fesseha, H. and Abebe, F. Degradation of Plastic Materials Using Microorganisms. Public Health – Open J., 2019, 4, 57–63. https://doi.org/10.17140/phoj-4-136
- Moubayed, N.M.S., Bhat, R.S., AlFarraj, D., Al Dihani, N., El Ansary, A., Fahmy, R.M. Screening and Identification of Gut Anaerobes (Bacteroidetes) from Human Diabetic Stool Samples with and without Retinopathy in Comparison to Control Subjects. Microbial Pathogenesis, 2019, 129, 88-92. https://doi.org/10.1016/j.micpath.2019.01.025
- Al Moussa, A.; Moubayed, M.N.S.; Al Jaloud, A.M.; Al Khattaf, F.S.; Dahmasha, N.D. Chicken feathers waste management by microbial as a sustainable and tool environmental friendly. Jep., 2021,12, 639-653. DOI: 10.4236/jep.2021.129039.
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution, 2018, 35, 1547-1549. https://doi.org/10.1093/molbev/msy096.
- Usha, R., Sangeetha, T., Palaniswam, M. Screening of Polyethylene Degrading Microorganisms from Garbage Soil. Libyan Agriculture Research Center Journal International, 2011, 2, 200-204
- Dharmalingam, S.; Hayes, D.G.; Wadsworth, L.C.; Dunlap, R.N.; DeBruyn, J.M.; Lee, J.; Wszelaki, A.L. Soil degradation of polylactic acid/polyhydroxyalkanoate-based nonwoven mulches. J. Polym. Environ., 2015, 23, 302–315. [CrossRef]
- Li, J.; Kim, H.R.; Lee, H.M.; Yu, H.C.; Jeon, E.; Lee, S.; Kim, D.H. Rapid biodegradation of polyphenylene sulfide plastic beads by Pseudomonas sp. Sci. Total Environ., 2020,720, 137616.
- Das, M.P.; Kumar, S. An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3 Biotech, 2015, 5, 81–86.
- Ho, B. T.; Roberts, T. K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach. Crit. Rev. Biotechnol., 2018, 38, 308–320. doi: 10.1080/07388551.2017.1355293
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv., 2019c, 39, 107457. doi: 10.1016/j. biotechadv.2019.107457
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE, 2018,13, (7) e
- Dang, T. C. H.; Nguyen, D. T.; Thai, H.; Nguyen, T. C.; Tran, T. T. H.; Le, V. H.; Nguyen, V.H.; Tran, X. B.; Pham, T.P. T.; Nguyen, T. G.; Nguyen, Q. T. Plastic degradation by thermophilic Bacillus sp. BCBT21 isolated from composting agricultural residual in Vietnam. Adv. Nat. Sci.: Nanosci. Nanotechnol., 2018, 9, 015014.
- Helen, A.S.; Uche, E.C.; Hamid, F.S. Screening for polypropylene degradation potential of bacteria isolated from mangrove eco-systems in Peninsular Malaysia. Int. J. Biosci. Biochem. Bioinform., 2017a, 7, 245–251
- Helen, A.S.; Uche, E.C.; Hamid, F.S. Screening for polypropylene degradation potential of bacteria isolated from mangrove ecosystems in peninsular Malaysia. Int. J. Biosci. Biochem. Bioinform., 2017b, 7, 245–251
- Jia, H.; Zhang, M.; Weng, Y.; Zhao, Y.; Li, C.; Kanwal, A. Degradation of poly butylene adipate-co-terephthalate by Stenotrophomonas sp. YCJ1 isolated from farmland soil. J. Environ. Sci. (China), 2021, 103, 50–58. https://doi.org/10.1016/j.jes.2020.10.001
- Dey, U.; Mondal, N.K.; Das, K.; Dutta, S. An approach to polymer degradation through microbes. IOSR J Pharmacy, 2012, 2, 385-388. ISSN: 2250-3013.
- Shalini, R. and Sasikumar, C. Biodegradation of Low Density Polythene Materials Using Microbial Consortium–An Overview. Int. J. Pharmaceu. Chem. Sci., 2015, 4, 507-514. ISSN: 2277-5005.
- Shalini, R. and Sasikumar, C. Biodegradation of Low Density Polythene Materials Using Microbial Consortium–An Overview. Int. J. Pharmaceu. Chem. Sci., 2015, 4, 507-514. ISSN: 2277-5005.
- Ghosh, S.; Qureshi, A.; Purohit, H.J. D-Tryptophan governs biofilm formation rates and bacterial interaction in P. mendocina and S. aureus. J. Biosci., 2019, 44, 3 DOI 10.1007/s12038-018-9841-7.
- Aarthy, M.; Puhazhselvan, P.; Aparna, R.; Sebastian, A.; Kuppuswami, M. Growth associated degradation of aliphatic-aromatic co-polyesters by Cryptococcus sp. MTCC 5455. Polym Degrad Stab., 2018, 152, 20–28. https://doi.org/10.1016/j.polymdegradstab.2018.03.021
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: microbial communities on plastic marine debris. Environment. Sci. Technol., 2013, 47, 7137-7146. https://doi.org/10.1021/es401288x.
- Aatikah, T.; Saira, S.; Atia, I.; Rida, B.; Nazia, J. Biodeterioration of Microplastics: A Promising Step towards Plastics Waste Management. Polymers, 2022, 14, 2275. https://doi.org/10.3390/polym14112275
- Ghatge, S.; Yang, Y.; Ahn, J.H.; Hur, H.G. Biodegradation of polyethylene: A brief review. Appl. Biol. Chem., 2020, 63, 27.
- Matjašiˇc, T.; Simˇciˇc, T.; Medvešˇcek, N.; Bajt, O.; Dreo, T.; Mori, N. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ., 2021, 752, 141959. [CrossRef] [PubMed]
- Sowmya, H.V., Ramalingappa, K.M., Thippeswamy, B. Biodegradation of polyethylene by Bacillus cereus. Adv. Polym. Sci. Technol. Int. J., 2014, 4, 28–32
- Montazer, Z., Habibi Najafi, M.B., Levin, D.B. Challenges with verifying microbial degradation of polyethylene. Polymers, 2020,12, 123.
- Sarwan, B., Acharya, A.D., Kaur, S., Pare, B. Visible light photocatalytic deterioration of polystyrene plastic using supported BiOCl nanoflower and nanodisk. Eurp. J., 2020, 134, 109793. [CrossRef]
- Alshehrei, F. Biodegradation of synthetic and natural plastic by microorganisms. J. Appl. Environ. Microbiol., 2017, 5, 8-19.

This work is licensed under a Creative Commons Attribution 4.0 International License.






