How to Cite | Publication History | PlumX Article Matrix
Formulation and Evaluation of Biopolymer Incorporated Nanogel of Nabumetone
Vrushali R. Jadhav1* and Kiran B. Erande2
and Kiran B. Erande2
1Department of Pharmaceutics, M.G.V’s Pharmacy College Panchavati, Nashik, Maharashtra, Affiliated to Savitribai Phule Pune University, Maharashtra, India.
2Department of Pharmaceutics, M.G.V’s S.P.H. College of Pharmacy, Malegaon Camp, Affiliated to Savitribai Phule Pune University, Maharashtra, India.
Corresponding Author E-mail: v.p.gotise10@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3187
ABSTRACT: The objective of this research was to improve the solubilization, dissolution rate, and thereby anti-inflammatory activity of the BCS class II drug Nabumetone (NSAID). A solid dispersion (SD) approach with Gelucire 50/13 was used to enhance the solubility. Microwave-induced fusion method was used for the development of SD, as it demonstrated a remarkable increase in the solubility and dissolution rate of pure drugs when compared to traditional solid dispersions. A number of parameters of the SD were evaluated in vitro, including solubility, dissolution, and Fourier Transformed Infrared Spectra obtained (FT-IR). The rate of dissolution was inclined to betterment with the drug: polymer ratio 1:0.5 showing drug release 99.60 ± 29. The objective of the work was to evolve a Nanogel for topical delivery of Nabumetone. The emulsification-diffusion method was used to create nanogel using the polymers Carbopol-940, Gelucire-50/13, and triethanolamine as gelling agents. Chitosan was added to the formulation as a biopolymer. Menthol was employed as a penetration enhancer. Using the spontaneous emulsification-diffusion method, six distinct formulations of Nabumetone-loaded Nanogel were successfully created and evaluated for particle size, zeta potential, thermodynamic stability, and rheology study. Using a cellophane membrane, the gels were examined for diffusion study further, and drug content, viscosity, spreadability, pH, and clarity were accessed. The formulation F4 batch had the best in-vitro drug release profile, with a 96.04% release rate over 270 minutes. Optimized gel F4 underwent a skin irritation study on Wistar rats and passed the test. The topical application of Nabumetone as Nanogel proved to be an effective approach to drug delivery.
KEYWORDS: Biopolymer; Cellophane membrane; Chitosan; Nabumetone; Nanogel; Solid dispersion
Download this article as:| Copy the following to cite this article: Jadhav V. R, Erande K. B. Formulation and Evaluation of Biopolymer Incorporated Nanogel of Nabumetone. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Jadhav V. R, Erande K. B. Formulation and Evaluation of Biopolymer Incorporated Nanogel of Nabumetone. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/47WxPZi |
Introduction
The therapeutic effectiveness of a pharmaceutical is determined by the drug molecules’ bioavailability and, finally, solubility. One of the key elements in reaching the necessary drug concentration in systemic circulation and displaying pharmacological response is solubility. Using drugs that are poorly soluble has a number of disadvantages, including higher doses, more frequent administration, and the possibility of side effects.1 Furthermore, for medications that are weakly water-soluble, the rate-limiting step in the process of absorption is their dissolution rate in GI fluids rather than the velocity with which pharmaceuticals diffuse across the gut wall. However, increasing the rate of dissolution and solubility of medications that are only marginally soluble in water is essential to increasing their oral bioavailability. Nabumetone has a melting point of 80–81°C, and a novel technique based on Microwave (MW) irradiation has gained acceptance as a means of producing solid dispersions (SD). MW radiation is electromagnetic radiation with wavelengths between 1 nm and 1 m that falls between infrared and radio frequencies within the scale of 0.3 to 300 GHz. Because MW can penetrate any substance2,3, it possibly transforms directly into heat within the material. The movement of molecules’ dipoles, which absorb microwave energy and transform it into heat, is to blame for this. As the transfer of energy is independent of heat diffusion, materials can be heated quickly and uniformly even when they are cold. Since many excipients are polymers and some melt at specific temperatures, this is crucial in the production of medication formulation. This method is frequently used for drying, polymeric cross-linking, and drug-polymer interaction, and it affects dosage forms by heating or electromagnetic fields, changing the structure of drug crystallites. The solubility and bioavailability of Nabumetone must be improved in order to increase its medicinal effectiveness.3 In order to achieve this goal, the current inquiry examines the potential of the melting, microwave fusion method with Gelucire50/13 as the carrier to produce SD for the improvement of Nabumetone’s solubility and dissolving rate. Transdermal drug delivery holds great promise, but it is a challenging technology to adopt for both systemic as well as local drug effects. Topical drug delivery is a development in pharmaceutical technology that motivates formulation scientists to investigate alternate pathways for the effective, efficient, and active delivery of drugs to the target site. The skin, one of the uncomplicated and easiest organs in the human body for topical administration, is the main route of topical formulation distribution.4 Due to the effectiveness of the drug diffusion through the layers of the skin and mucous membrane, topically administered medications show limited effect at the application site. These topical drug delivery systems are frequently engaged to treat localized skin conditions or in locations where other medication administration routes are inefficient. Through the topical method, the drug was delivered to the skin surface in an absorbable drug formulation.5 The primary interest of transdermal delivery of drugs is, it avoid first-pass metabolism6, which reduces risk and issues related to intravenous therapy such as changes in pH, presence of enzymes, gastric emptying hold up, etc.7 The bulk of the human body is covered by skin, making it one of the most attainable organs for topical drug delivery.8,9 The properties of colloids and gels, such as a micro heterogeneous structure, high surface-volume ratio, and microscopic diameters, are shared by nanogels, which are smaller counterparts of hydrogels.10,11 Nanogels are supple, stable, and swollen in exemplary solvents. The solids create a 3-D network with a nanoscale size, resulting in the bioconjugation of active targeting sites and a large surface area. It is made up of a few small solid parts that are mixed with polymers that are spread throughout a lot of liquid. A Nanogel matrix can hold and safeguard drug molecules in hydrogel by physical and chemical cross-linking polymer at the nanostructured level.12,13 Nanogel has a particle size range of 10–100 nm. The main influence of topical NSAIDs is the lack of remarkable side effects associated with systemic delivery of NSAIDs, typically in older individuals. In actuality, greater renal and gastrointestinal damage has been related to oral NSAID medication. Indications recommend that topical dosage forms can provide therapeutic drug concentrations in localized tissues by preventing systemic toxicity and maintaining low serum drug levels. The goal of this study was to evolve and test a Nabumetone Nanogel that offers delayed release, increases the drug’s time of contact, and hence improves bioavailability.
Material and methods
Nabumetone was a gift sample from IPCA Labs Limited, Ratlam. Gelucire50/13 polymer gift sample from Modern Industries c-74, MIDC Malegaon-sinner, dist. Nashik, Carbopol-940, and Glycerol from Research-lab Fine Chem Industries Mumbai. Tween- 80 and Methyl Paraben was procured from Modern Industries c-74, MIDC Malegaon-Sinnar, Nashik, and Chitosan was procured from Research-lab Fine Chem Industries Mumbai. Propylene Glycol was procured from Modern Industries c-74, MIDC, Malegaon-Sinnar, dist. Nashik. Triethanolamine was procured from Sigma Enterprises Makhmalabad Naka, Panchavati, Nashik.
Method for solid dispersion
Method for Solid Dispersion by Microwave-Induced Fusion:
By using microwave irradiation, a solid dispersion of Nabumetone and Gelucire50/13 in a 1:0.5 weight-to-weight ratio was created. This substance was given the name MSD. In a residential microwave oven, 1gm of a blend of drug and polymer (1g) was placed in a beaker and microwaved for about five minutes at the power of 600 W. (mod. Catalyst 2R, Catalytic Systems). Then, to achieve consistent particle size, SDs were mashed in a mortar made up of glass and put through a sieve of mesh size 100. 2,3,17.
Solubility study18
In the USP dissolving medium phosphate buffer pH 6.8 at 37-+ 0.2 o C, the apparent solubility of Nabumetone, a physical mixture or blend, and SDs made by microwave-induced fusion technique were determined. Weighing the equivalent of 10 mg of Nabumetone into each vial is the approach for figuring out how soluble the drug is. Following this, 10 ml of phosphate buffer (pH 6.8) was introduced to all of the vials containing Nabumetone, and they were shaken to equilibrium at 37-+ 0.2 o C for 24 hours. Each vial has a 1ml aliquot that was filtered via a 0.45 um Millipore filter. Each sample’s Nabumetone content was examined using a UV spectrophotometer at 271 nm.
Dissolution studies3,19
Nabumetone, a physical mixture, and solid dispersions were studied for their ability to dissolve in buffer pH 6.8 USP dissolution medium 900 ml containing potassium dihydrogen phosphate and sodium hydroxide at a temperature of 37-+ 0.5 oC. using a dissolution test apparatus USP type II (TDT 08 plus- ELECTROLAB, Mumbai, India). 10, 20, 30, 40, 50, 60, 70, 80, and 90-minute intervals. 5-ml samples were divided into aliquots, filtered using a 0.45-um Millipore filter, diluted, and then subjected to a UV spectrophotometer analysis at 271 nm. The estimated average values of cumulative drug release were utilized to produce the drug release curves after each sample’s dissolution rate was assessed in triplicate.
Characterization of Solid Dispersions
Study using Infrared Spectroscopy
Diffuse reflectance scan (DRS) sampling was used to produce the FTIR spectra of Nabumetone, Gelucire50/13, physical blend, and microwave-generated SDs using an FTIR (IR affinity 1 shiatsu, Japan) (1 mg sample in 100 mg Kerr). The resolution was 1 cm-1, with a 4000-400-1 cm scanning range.
Method: for nanogel preparation
Emulsion- Solvent Diffusion Method20,21
The Nanogel is created using a modified version of the emulsion solvent diffusion process. There are 4 steps in it.
Step I: With stirring, a precisely weighed amount of the medication is dissolved in glycerol and Tween-80 (organic phase).
Stage II: By dissolving Carbopol-940 in water and boiling it for 20 minutes during magnetic stirring, the aqueous phase is produced in the second stage. After that, the drug is solicited in an ultrasonic system for 10 minutes.
Step III: The drug portion is added dropwise into the aqueous phase throughout the course of high-speed homogenization with a Probe Sonicator (ultrasonic processor) for 30 minutes at 6000 rpm. When the emulsion is dispersed into nanodroplets by the ultrasonic homogenizer, an o/w emulsion is produced.
Phase IV in this step involves adding triethanolamine while stirring continuously to an o/w emulsion that has been homogenized for an hour at 8000 rpm.
Table 1: Manufacturing formula for Nanogel.
|
Ingredient (%w/w) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Physical mixture (Nabumetone+Gelucire50/13) |
1 |
1 |
1 |
1 |
1 |
1 |
|
Carbopol-940 |
0.5 |
1.0 |
1.5 |
— |
— |
— |
|
Chitosan |
— |
— |
— |
2 |
1.5 |
1 |
|
Glycerol |
10 |
10 |
10 |
10 |
10 |
10 |
|
Tween 80 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Methyl Paraben |
0.15 |
0.15 |
0.15 |
0.15 |
0.15 |
0.15 |
|
Menthol |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
|
Triethanolamine |
1 |
1 |
1 |
1 |
1 |
1 |
|
Water |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
Characterization of Nanogel
FTIR study (Fourier transforms infrared spectroscopy)22,23
Shimadzu’s S 8400 spectrophotometer was used to record the IR spectra of Nabumetone and its excipients. The sample was made ready via the KBr disc process and assessed in the transmission system. The spectrum was computed throughout the 4000-400 cm frequency band. The proper functional groups in the Nabumetone structure were then compared to the peaks in the spectra. Nabumetone and Gelucire50/13 solid dispersion FTIR analysis.
Physical appearance
Visual examination of the prepared nanogel formulations was done to check for organoleptic properties like color, homogeneity, texture, and separation of phases.
pH Determination24
To gauge the pH of the produced mixture, a digital pH meter was employed. The procedure entailed adding nanogel to a 250 mL beaker, mixing the mixture with a pH meter, and logging the pH meter readings. Two times the process was done with the exact same mixture. Three times, the pH of each created mixture was assessed using the same methodology.
Measurement of particle size of the formulation25
Malvern master size 2000 MS was used to calculate the average size of the chosen Nanogels. It was noted the mean particle size (313 nm).
Zeta potential measurement26
At the Savitribai Phule Pune University in Pune, the stability of the synthesized Nanogel was assessed by measuring its zeta potential, which was found to be between the intended range and 47 mV.
Drug Content Determination27
Nabumetone was brought out from 1 g of a gel constitution using 50 ml of pH 6.8 phosphate buffer for the estimation of the drug in gel and the resulting mixture was then passed through a membrane filter (pore size 0.45 um). From the above filtrate, 2 ml was pipetted out and adjusted in volume up to 10 ml. At 271 nm, the sample’s absorbance was measured spectrophotometrically. The calibration curve was used to estimate the Nabumetone concentration.
Rheology study of Nanogel28,29
A rotating viscometer of the Brookfield type with spindles 61 and 63 was used to measure the viscosity of the various formulations of Nanogel at 50 rpm for one minute at 250 C.
Scanning Electron Microscopy (SEM)
Scanning electron microscopy was used to examine the shape and surface morphology of the Nanogel created with optimal settings.
Extrudability28
A hardness tester was used to conduct the extrudability test. Aluminum collapsible tubes were filled with a 5-gm dose of Nanogel. The plunger is tested to ensure that the tube is held securely. 1 g/cm2 of force was applied for 30 seconds. The amount of Nanogel that was then extruded from the tube was measured, and the process was repeated three times.
Extrudability is calculated as follows: Area/Applied weight (in grams) to extrude the Nanogel form tube (in cm2)
Spreadability29
Glass slide and wooden block measuring tools were used to determine it. The time it took for the upper slide to completely disengage from the fixed slides was timed after adding a weight to the pan of about 20g.
After that, spreadability was determined using the following formula: S= M. L/T
S stands for spreadability, M for weight to the upper slide, and L for glass slide length.
T is the amount of time needed to completely separate the slides from one another.
In-vitro release studies:30, 31
The Franz Diffusion Cell, which was a cylindrical glass tube with openings at either end, was used to gauge how quickly the formulation released the medicine. A cellophane membrane that had previously been soaked in the medium for 24 hours was coated with 1 g of gel, which is equal to 10 mg of Nabumetone, and bonded to one end of the tube. The entire setup was adjusted so that the surface of the diffusion medium, which was 100 ml of pH 6.8 phosphate buffer in a 100 ml beaker, was just touched (1-2 mm deep) by the lower end of the tube holding the gel. The assembly was set up on a magnetic stirrer-equipped thermostatic hot plate, which was kept at a temperature of 37 o C. 5 ml of samples were taken at various times during the 24 h of stirring the mixture with a magnetic bar at 100 rpm. Using a new phosphate buffer (pH 6.8), this 5ml was diluted up to 10ml, and samples were examined for Nabumetone at 271 nm in a UV-Vis spectrophotometer.
Skin Irritation study:32, 33
Six Wistar rats were used for the experiment. The animals were allowed to acclimatize for 7 days after procurement. Temperature (22±2℃) and humidity (45-55%) were controlled. The animals were kept for 12 hours in a light/dark cycle. One day before the application of the test formulation, hair removal was done from the dorsal skin of the rats by using hair removal cream. The test formulation was applied uniformly over the uncovered area of the dorsal skin. The test formulation was held in contact with the skin with a porous gauze dressing and non-irritating tape. The test site was further covered in a suitable manner to retain the gauze dressing and test formulation. Normal saline was applied to the skin of animals belonging to the control group. Through the one-day exposure period rats were caged separately to avoid ingestion of the test chemical by other rats in the cage. Rats were observed instantly after dozing at least one time during the first half hour and intermittently during the first 24 hours period with special caution during the first two to six hours after the starting of the exposure period. Additionally, the application site was observed at 24-, 48-, and 72-hour intervals after the skin was exposed. Erythema was graded on a scale of 0 to 4 indicating No erythema to severe erythema observed.31, 32
Results and Discussion
Solid Dispersions Evaluation
UV- Spectrophotometric study
The UV spectrum of Nabumetone is shown in Figure No. 1.
 |
Figure 1: UV-Spectrum of Nabumetone
|
Standard calibration curve of Nabumetone in Phosphate buffer (pH 6.8).
Table 2 shows the absorbance of a standard solution of Nabumetone in phosphate buffer pH 6.8 (n=3) The regression value was found to be 0.9945 as shown in Figure No.2.
Table 2: Absorbance of Nabumetone (Serial dilutions) in pH 6.8 Phosphate buffer
|
Sr. No. |
Concentration (ug/ml) |
Absorbance value and RSD (Relative Std. Deviation%) |
|
1 |
0 |
0.000 0.000 |
|
2 |
5 |
0.184 0.134 ±0.016 |
|
3 |
10 |
0.278 0.278±0.02 |
|
4 |
15 |
0.384 0.384±0.017 |
|
5 |
20 |
0.496 0.496±0.016 |
|
6 |
25 |
0.635 0.635±0.021 |
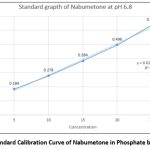 |
Figure 2: Standard Calibration Curve of Nabumetone in Phosphate buffer pH 6.8. |
Solid dispersion of Nabumetone: Gelucire50/13 by microwave-induced procedure indicated the most improved solubility among all SDs with an indication of solubility as 0.530+0.10 mg/ml.
Table 3: Ratio Optimization of Nabumetone and Gelucire50/13.
|
Drug: polymer ratio |
Solubility reading (mg/ ml) |
|
1:0.25 |
0.0311±0.001 |
|
1:0.5 |
0.0530±0.010 |
|
1:0.75 |
0.0379±0.050 |
|
1:1 |
0.0372±0.018 |
All results were estimated as mean + standard deviation, n=3. Indicating p<0.001.
The in vitro dissolving characteristics of Gelucire50/13-prepared solid dispersions of the pure medication. Solid dispersions made using the microwave technique were compared to the dissolving profile of Nabumetone. At the conclusion of the Nabumetone experiment, the microwave approach produced solid dispersion with a maximum cumulative release of 99.60 29%. These results indicate a substantial increase in the rate of Nabumetone dissolution from microwave-prepared solid dispersion. Table No.4 and Figure No.3 exhibit the % drug release data in USP dissolution medium (pH 6.8 buffer) at 10 to 90 minutes. The microwave-produced SDs indicated a substantial rise in the dissolving rate from 10 to 90 minutes.
Table 4: Percentage Release of Nabumetone, from Optimized Solid Dispersions.
|
Physical mix 1:0.5 (Nabumetone+Gelucire50/13) |
Drug Release Solid Dispersion |
|
Time (min) |
Drug Release |
|
10 |
37.8±0.05 |
|
20 |
40.6±1.00 |
|
30 |
46.2±0.04 |
|
40 |
55.7±0.06 |
|
50 |
67.2±0.75 |
|
60 |
70.1±0.04 |
|
70 |
76.9±0.95 |
|
80 |
88.9±0.85 |
|
90 |
99.60±29 |
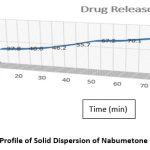 |
Figure 3: Dissolution Profile of Solid Dispersion of Nabumetone and Gelucire50/13. |
From the FTIR spectra, it has been observed that there is no molecular shifting in the functional group as if that of a pure drug specified in pharmacopeia hence there is a compatibility between the drug and polymer. The separate FTIR spectra of the pure drug Nabumetone as well as polymer Gelucire50/13 as a spectrum of the mixture of the drug and polymer and optimized formulation F4 are as follows
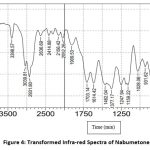 |
Figure 4: Transformed Infra-red Spectra of Nabumetone. |
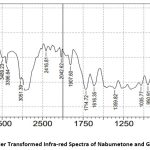 |
Figure 5: Fourier Transformed Infra-red Spectra of Nabumetone and Gelucire50/13. |
Table 5: Interpretation of FTIR Spectra.
|
Functional groups |
Actual IR Range |
Observed IR Range Nabumetone |
Observed IR Range Nabumetone+Gelucire50/13 |
|
C-H Stretching |
2970-2850 |
3039.81-2931.80 |
2931.80 |
|
C=O Carbonyl group |
1750-1705 |
1714.72-1703.14 |
1703.14 |
|
Ether O-CH3 |
2850-2815 |
2837.12 |
2837.12 |
|
C=C-C Aromatic ring stretching |
1680-1620 |
1616.35-1614.42 |
1614.42 |
|
N-H Amine |
3390-3400 |
—————– |
3396.64 |
The IR spectra observed lie in the range of standard values of corresponding functional groups. Hence it confirms that there is no unwanted interaction between the drug and the polymer used.
Characterization of Nanogel
Physical appearance
Table 6: Physical Appearance of the Nanogel Formulation
|
Sr. No |
Formulation code F1 to F6 |
Color |
Phase Separation |
Grittiness |
|
1 |
F1 |
Transparent |
None |
None |
|
2 |
F2 |
Transparent |
None |
None |
|
3 |
F3 |
Transparent |
None |
None |
|
4 |
F4 |
Transparent |
None |
None |
|
5 |
F5 |
Transparent |
None |
None |
|
6 |
F6 |
Transparent |
None |
None |
pH Determination
The pH of all test formulations from F1 to F6 was determined and enlisted in Table No. 8
Particle size analysis and Zeta potential
The particle size determination of the nanogel was carried out by using a particle size analyzer (Zetasizer) F4 Bach (313 nm). The zeta potential of the Nanogel F4 was measured at SPPU University in Pune to assess the stability of the formula. The particle size determination of the nanogel was carried out by using a particle size analyzer (Zetasizer) F4 Bach (313 nm). The stability of the formulated Nanogel was evaluated by SPPU University, Pune measuring the zeta potential of the Nanogel F4 (desired range ± 47 mV)
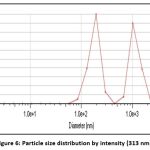 |
Figure 6: Particle size distribution by intensity (313 nm). |
Table 7: Particle size analysis of Nanogel containing Nabumetone
|
Formulation code F1 to F6 |
Particle size analysis (nm) |
|
F1 |
354.65 |
|
F2 |
350.40 |
|
F3 |
328.59 |
|
F4 |
313.65 |
|
F5 |
447.03 |
|
F6 |
465.05 |
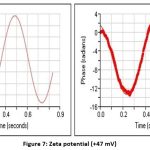 |
Figure 7: Zeta potential (+47 mV). |
Homogeneity
All of the test formulations (F1-F6) indicated good homogeneity no unevenness was observed. Gel formulations turned out to be transparent and were free from the grittiness of Particles, the appearance of gel aggregates, foreign particles or impurities, as well as phase separation. Results are indicated in Table No. 8.
Rheology study of Nanogel
All Nanogel formulations were introduced to a Brookfield viscometer and viscosity (in cps) was measured by dropping a cone attached to a holding rod so that it ought to land in the middle of the Nanogel-filled glass cup. The outcomes are displayed in Table No. 8.
Spreadability
The spreadability range for various nanogel formulations F1-F6 indicated good spreadability, the nanogel was easily spreadable, as demonstrated by F1–F6. The outcomes are displayed in Table No. 8.
Extrudability
For a good Nanogel formulation, it should be extruded easily from the container. The Extrudability of all formulations was found to be good. The results are shown in Table No. 8.
Drug content
The drug content of all gel formulations from F1 to F6 is shown in Table No. 8. There is not much difference in the drug content of each formulation the higher drug content in the F4 batch and less drug content in the F6 batch so, the effect of polymers ratios is less considerable here.
Table 8: Evaluation of test formulation batches of Nanogel.
|
Formulation Code F1 to F6 |
Homogeneity |
pH |
Spreadability (cm) |
Extrudability |
Drug Content (%) |
Viscosity (cps) |
|
F1 |
Homogeneous |
5.9 |
25.2 |
245 |
80.4 |
3260 |
|
F2 |
Homogeneous |
6.3 |
24.3 |
254 |
75.5 |
3358 |
|
F3 |
Homogeneous |
6.4 |
22.8 |
268 |
91 |
3280 |
|
F4 |
Homogeneous |
6.6 |
28 |
277 |
94.5 |
3488 |
|
F5 |
Homogeneous |
6.8 |
18.4 |
260 |
86 |
3345 |
|
F6 |
Homogeneous |
6.2 |
17.6 |
275 |
69.5 |
3403 |
Scanning Electron Microscopy (SEM)
Determination of surface morphology and shape of nanogel was conducted by ZEISS EVO. US scanning electron Microscope. Evaluation was done under the magnification of 25KX and 30KX respectively (as shown in Fig. 8a. and b.) and energy (EHT) Electron High Tension 25kV was utilized. It was observed that formulation F4 exhibits 200 nm size nanogel particles. The SEM images show that there is no breakage of nanogel. The results are shown in Fig. 8 a. and b.
 |
Figure 8: a and b. SEM images of F4 batch formulation. |
In – Vitro diffusion studies and Kinetics
The drug release from the nanogel was studied by the Franz diffusion cell method. The in vitro release profiles of Nabumetone from Nanogel are shown in Table No.9. Corresponding graphical presentation of in-vitro drug diffusion of all nanogel formulations is shown in Fig. 9. The cumulative percentage release of Nabumetone Nanogel varied depending on the drug-polymer ratio for 24 hrs. The Zero order release kinetics of various formulations are shown in Fig. 10 and R2 values of all formulations by applying various kinetic models are reported in Table 10.
Table 9: In–Vitro diffusion studies.
|
Time (Minutes) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
30 |
7.70 |
7.42 |
6.16 |
7.98 |
7.14 |
7.84 |
|
60 |
10.5 |
10.08 |
10.50 |
10.92 |
8.82 |
11.06 |
|
90 |
14.98 |
12.18 |
11.62 |
22.82 |
14.98 |
16.66 |
|
120 |
24.64 |
16.94 |
13.44 |
34.44 |
22.54 |
21.14 |
|
150 |
39.20 |
32.34 |
18.06 |
46.06 |
38.22 |
36.54 |
|
180 |
60.06 |
38.50 |
33.60 |
60.34 |
58.66 |
57.54 |
|
210 |
70.84 |
57.50 |
46.62 |
75.46 |
73.64 |
67.34 |
|
240 |
88.06 |
78.54 |
75.46 |
91.00 |
88.34 |
80.35 |
|
270 |
89.46 |
83.30 |
80.50 |
96.04 |
91.84 |
88.06 |
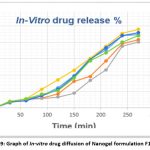 |
Figure 9: Graph of In-vitro drug diffusion of Nanogel formulation F1 to F6 |
Table 10: R2 Values of different formulations on applying various kinetic models.
|
R2 Values |
ZERO-ORDER |
1ST ORDER |
HIXON |
HIGUCHI |
KORSEMAYER PEPPAS |
|
Formulation |
|||||
|
F1 |
0.9567 |
0.9467 |
0.9015 |
0.8007 |
0.5067 |
|
F2 |
0.9225 |
0.9123 |
0.855 |
0.7455 |
0.4574 |
|
F3 |
0.8613 |
0.8211 |
0.7904 |
0.673 |
0.3994 |
|
F4 |
0.9827 |
0.9522 |
0.9034 |
0.8451 |
0.5529 |
|
F5 |
0.9514 |
0.9213 |
0.8932 |
0.7871 |
0.4904 |
|
F6 |
0.9574 |
0.9182 |
0.9085 |
0.8007 |
0.5084 |
 |
Figure 10: Zero order Kinetic treatment for Nabumetone Nanogel and diffusion kinetic model for all formulations. |
Skin Irritation study
Optimized formulation F4 was tested for the assessment of skin irritation in Wistar rats and animals treated with the formulation did not develop any erythema after application of the formulation (The study was carried out for seven days). Observations from the study are shown in Table No.11 and Fig. No. 11.
Table 11: Skin Irritation Study.
|
Control Group |
||||||||||||
|
Time/Day |
Day 1 |
Day |
||||||||||
|
30 min |
60 min |
120 min |
180 min |
240 min |
300 min |
2 |
3 |
4 |
5 |
6 |
7 |
|
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Mean |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Formulation Group |
||||||||||||
|
Time/Day |
Day 1 |
Day |
||||||||||
|
30 min |
60 min |
120 min |
180 min |
240 min |
300 min |
2 |
3 |
4 |
5 |
6 |
7 |
|
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Mean |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
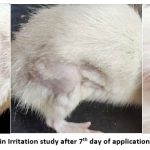 |
Figure 11: Result of Skin Irritation study after 7th day of application (No erythema is seen) |
Conclusion
Successful creation of a nanogel formulation including Nabumetone and Biopolymer yielded an excellent and superior carrier for the transdermal/topical application. SEM has made this new method of drug delivery popular. The created Nanogel formulation was optimized regarding homogeneity, particle size, pH, drug content, in-vitro drug diffusion, spreadability, and viscosity and tested for skin irritation. When administered via the dermal route, the therapy’s plasma levels are maintained for the duration of a single dose while avoiding the drawbacks of oral administration. The test medication release rate for solid dispersion is 99.60%. As a result, the drug’s solubility and bioavailability both increased. Due to incomplete gel formation in the early time period, each formulation’s initial release rate was very quick; however, after complete gel formation, the release rate became slow. Stability enhances the loading of water-insoluble drugs in hydrophilic gel bases for the long term. The test formulations were released in vitro to gauge drug release from Nanogel. In-vitro tests with formulation F4 showed a maximum release of 96.04 % in 270 minutes. The test formulation did not produce erythema when tested on Wistar rats for seven days during the skin irritation study. Based on the assessment parameter results of the trial batches, batch F4 was determined to be the optimized batch, and an experimental design was created. Nabumetone Nanogel can therefore be utilized as a topical delivery system to treat arthritis pain.
Acknowledgment
The authors would like to acknowledge M.G.V’s Pharmacy College, Panchavati, Nashik, Maharashtra, India- 422003 for providing the necessary facilities to perform this research work. The authors would also like to acknowledge Savitribai Phule Pune University for providing the grant for this research work under ‘ASPIRE’- a minor project scheme by IQAC, SPPU.
Conflict of Interest
The authors declare no conflict of interest
Funding Source
Savitribai Phule Pune University provided a grant for this research work under ‘ASPIRE’- a minor project scheme by IQAC, SPPU.
References
- Dongfei L, Yifan G, Yue T, Yubing Y, Qing Z, Rui L. Solid lipid nanoparticles for transdermal delivery of diclofenac sodium: preparation, characterization and in vitro studies. Jour. of Microencap. 2010; (27) 726- 734
CrossRef - Laxmikant Z, Sanjay B. Microwave Induced Solid Dispersion as a Novel Technique for Enhancing Dissolution Rate of Repalinide, Adv in Pharmac. and Pharm. 2013;1(2): 95-101.
CrossRef - Hyma P, Renuka R, Rajani C.H, Kumar S.D.S. Formulation and Evaluation of Solid Dispersions of Nabumetone. Jour of Drug Dev and Ther. 2013; 3(4), 15-19.
CrossRef - Thomas H, Ola S, Peter W, Hans W, Kjell A. U, Anders E. Nabumetone Therapeutic Use and Safety Profile in the Management of Osteoarthritis and Rheumatoid Arthritis. Drugs. 2004; 64 (20) 2315-43.
CrossRef - Rani J. R, Gouri R. D, Kanchan P. U, Suparna S. B, Rohit T. D. A Comprehensive Review on Emulgel: A New Approach for Enhanced Topical Drug Delivery, Int Jour of Mod Pharm Res. 2021;5(3)222-223.
- More A., Abdul W. A. Development and Characterization of Nanoemulsion Gel for Topical Drug Delivery of Nabumetone. IJPPR Oct-2016; 7 (3), 126-157.
- Saroha K, Sarabjeet S, Aggarwal A, Nanda S. Transdermal Gels – An Alternative Vehicle for Drug Delivery. Int Jour of Pharm, chem and Bio. Sci. 2013; 3(3),495-503.
- Sushma G, Pravalika T, Renu S. B, Priyanka P, Dr. Vishnu P. P, Dr. Sharma J.V.C. Emulgel: A Novel Approach to Topical Drug Delivery. Int Jour of Pharma and Bio. Sci.2013; 4(1); 848-850.
- Kute S. B, Saudagar R. B; Emulsified gel A Novel approach for delivery of hydrophobic drugs: An overview. Jour of Adv Pharm. Edu & Res. 2013; 3(4); 368-373.
- Iordana N, Alina G. R, Alina D, Loredana E. N, Aurica P. C. Basic Concepts and Recent Advances in Nanogels as Carriers for Medical Applications. Drug Delivery. 2017;24(1), 539-57.
CrossRef - Basha B.N, Prakasam K, Goli D. Formulation and evaluation of gel containing fluconazole-antifungal agent. Int J Drug Dev Res. 2011;3(4):109-28.
- Sushma G, Pravalika T, Renu S. B, Priyanka P, Dr. Vishnu P. P, Dr. Sharma J.V.C. Emulgel: A Novel Approach to Topical Drug Delivery, Int Jour. Pharm. Sci Rev. Res. 2021; 67(1);142-147.
CrossRef - Vats S, Saxena C, Easwari T.S, Shukla V. K. Emulsion Based Gel Technique: Novel Approach for Enhancing Topical Drug Delivery of Hydrophobic Drugs. Int Jour. Pharm. Res. Scholar, 2014;3(2); 650.653.
- Talele S, Nikam P, Ghosh B, Deore C, Jaybhave A, Jadhav A. A Research Article on Nanogel as Topical Promising Drug Delivery for Diclofenac Sodium. Ind J of Pharma.Edu. and Res. 2017; 51(4S);5680-5807
CrossRef - Kriplani P, Sharma A, Aman, Pun P, Chopra B, Dhingra A. Formulation and Evaluation of Transdermal Patch of Diclofenac Sodium, Global J of Phar & Pharm Sci. 2018;4(5); 1-4.
CrossRef - Muniraj S N, Yogananda R, Nagaraja T S, Bharathi D. R. Preparation and Characterization of Nanogel Drug Delivery System Containing Clotrimazole an Anti-Fungal Drug. Indo-American Jour. of Pharm Res. 2020; 10(7); 1013- 22.
- Raju S, Pravin B, Vivekanand C, Sanjay B, Laxmikant Z. Preparation and Characterization of microwave-assisted Candesartan Cilexetil Solid Dispersion. Jour. of Pharm. Res. 2013; 9-19
- USP2-NF24, Volume No. 31 (1) Page 63.
- Costa P, Loba JMS. Modeling and comparison of dissolution profiles. Eur. Jour. Pharm. Sci. 2001; 13:123-133
CrossRef - Fedora G, Gaetano R, Rita M, Maria A. O, Elisabetta M, Michele D. L. Gel Formulation of Nabumetone and a Newly synthesized Analog: Microemulsion as a photoprotective Topical Delivery System, Pharmaceutics 2020, 12(5), 423; 1-14.
CrossRef - Sandeep K, Neeraj D, Ruma S, Gaurav B. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bio Nano Science (2012). 2(4);1-27
CrossRef - Pankaj K, Chander M, Mara K. S. U. S, Monica G. Physiochemical characterization and release rate studies of solid dispersions of ketoconazole with pluronic F127 and PVP K-30, Iran. Jour. Pharm. Res.10 (2011) 685–694.
- More A, Ambekar A.W. Development and Characterization of Nanoemulsion Gel for Topical Drug Delivery of Nabumetone, IJPPR Human. 2016; 7(3): 126-157
- Hetty L. M, Kasmirul R, Sinaga M. Formulation and Evaluation of Miconazole Nitrate Nanoemulsion and Cream. Asian J Pharm Clin Res, 2018, 11(3);319-321.
CrossRef - Shirsand S. B, Para M.S, Nagendrakumar D, Kanani K.M., Keerthy D. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system, Int J. Pharm. Invst. 2012,2(4); 201- 208.
CrossRef - Bhatt P. Madhav S. A. Detailed Review on Nanoemulsion Drug Delivery System. Inter. Jour. of Pharm. Sci and Res. 2011; 2 (10):2482-2485.
- Amit G, Mishra A.K, Amrit K. S, Verruchi G. Formulation and evaluation of topical gel of diclofenac sodium using different polymers. Drug invent. today. 2010;2(5):250-3.
- Pranuti D. P, Susmita B. P, Prasanna S. P, Dr. Vijay R. S. Design and development of nanogels containing Carbopol and hydroalcoholic extract of Pterocarpus marsupium. World jour of pharm. Res. 2018; 7(19):790-801.
- Singh K, Mishra A, Singh A. Preparation, characterization and in vitro release profile of ketoconazole loaded chitosan nanoparticle, Int. J. Med. Pharmaceut. Sci. 2016, 6 (5);1-8.
CrossRef - Balata G, Mahdi M, Bakera R.A. Improvement of solubility and dissolution properties of clotrimazole by solid dispersions and inclusion complexes, Indian jour. of Pharm. Sci. 2011 73(5); 517 -526.
CrossRef - Thombare N.A; Niphade P.S; Ahire E.D.; Kshirsagar S.J; Formulation Development and Evaluation of Microemulsion Based Lornoxicam Gel, BBRA, March 2022, 19(1); 69-80.
CrossRef - Gupta R, Das G. G. Formulation Development and Evaluation of Anti-inflammatory Potential of Cordia obliqua Topical Gel on Animal Model, Pharmacog. Jour. 2017; 9(6); 93-98.
CrossRef - Rajasekaran A, Arulkumaran G, Arivukkarasu R. Formulation and evaluation of topical herbal gel for the treatment of arthritis in an animal model, Braz. Jour. of Pharma. Sci, 2016, 52(3); 494-507.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






