How to Cite | Publication History | PlumX Article Matrix
Isolation and Production of Cellulase from Bacteria Using Agro Waste
Nikita R. Chavda* , Priti. H. Patel
, Priti. H. Patel , Roshani K. Chaudhary
, Roshani K. Chaudhary
Department of Biotechnology, Ganpat University, Mehsana, Gujarat, India.
Corresponding Author E-mail:nikitachavda276@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3192
ABSTRACT: The development of appropriate procedures for the efficient treatment and utilization of wastes containing cellulose as an inexpensive carbon source has grown to be of substantial economic relevance. Cellulase enzyme, which is known to be produced by bacteria is responsible for degrading cellulose. Thus, isolation of Bacteria producing cellulase was performed using soil sample that were identified as Bacillus subtilis, Pseudomonas aeruginosa, Enterobacter cloacae using CMC medium. The medium for fermentation was optimized for maximum cellulase to be produced by the potential isolate. Various parameters like the time of Incubation, temperature, pH, nitrogen sources and carbon sources, were considered for optimization. The culture condition was optimized and found to be 40°C at pH 7 with maximum activity in the presence of ammonium sulphate and lactose as nitrogen and carbon sources respectively. Amongst these isolates the maximum cellulase activity was shown by Enterobacter cloacae followed by Pseudomonas aeruginosa and Bacillus subtilis by comparative study. The supplement for the medium was various agricultural waste added as an alternate source of carbon to produce cellulase. The medium with the presence of rice husk (1.76 IU/ml), followed by wheat husk(1.51 IU/ml) and castor seed waste (0.65 IU/ml), had the highest cellulase activity. Thus, this work aimed to compare the potential of all the above-mentioned isolates to use agro-waste for production of cellulase at optimized parameters.
KEYWORDS: Agro waste; Bacteria; Cellulase; CMC; optimization; production
Download this article as:| Copy the following to cite this article: Chavda N. R, Patel P. H, Chaudhary R. K. Isolation and Production of Cellulase from Bacteria using agro waste. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Chavda N. R, Patel P. H, Chaudhary R. K. Isolation and Production of Cellulase from Bacteria using agro waste. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/4aj3N3U |
Introduction
The planet is abundant with cellulose.1. It is one of the plentiful renewable resources in nature and is primarily found during the process of photosynthesis in plants of the terrestrial environment.2,3. Cellulase is the enzyme that degrades cellulose. Several bacteria and fungi are found to produce this enzyme 4–7.
Cellulose is mostly found in the cell walls of terrestrial plants. The microfibrils of plants present in cellulose are the source present in plants. These microfilaments are responsible for providing strength to the plants. Utilization of cellulosic biomass is done enormously worldwide but still there are some raw materials or waste materials which need to be exploited and used efficiently. [1]
It is important to develop the technique for the economic use of this cellulose that is profitable. The Synergistic approach of three enzymes helps in the complete hydrolysis of cellulose, namely, carboxymethylcellulose (CMCase) or endoglucanase, cellobiohydrolase, and beta-glucosidases.8
Various Bacteria with higher growth rates possess good potential to produce cellulase as compared to fungi. However, this approach of using bacteria for the production of cellulase is not used widely. Indeed, the use of fungi is a more common way for producing cellulases. It was reported that there are several bacterial genera like Bacillus, Micrococcus,7, Pseudomonas,9. Cellulomonas, Cellvibrio sp. has cellulolytic properties. It is necessary to control various parameters for maximum cellulase production during optimization. These parameters work in the relationship and are majorly responsible for an overall yield of cellulase which includes pH, inoculum size, temperature, carbon and nitrogen sources, inducers, etc. 7.
There are various sectors releasing waste materials containing cellulose like industries, agriculture and municipal. Due to high processing costs, this trash keeps building up and is used inefficiently, which hinders solid waste management. 10. One of the many types of carbohydrates that can be found in nature, cellulose is the primary structural element of the plant cell wall. It is made up of glucose residues made up of beta 1, 4 linkages. 11. Cellulose is an inexpensive carbon source and it is extremely essential to develop a process that helps in the proper utilization of cellulose at an economic level. Cellulase enzyme helps to hydrolyze this polymer and release glucose 12.
Cellulose is released from various wastes released from industries, agricultural and urban sewage and this source of carbon helps in the production of methane by the process of anaerobic digestion. Agricultural waste includes residues of crops, excreta of animals, wastes from crop processing industries and activities of forestry including products of wood. It is now a requisite to effectively use these wastes as carbon sources and not consider them valueless. 13
The utilization of cellulase can be done in various sectors like the preparation of washing powders, extracting juice from fruits and vegetables and processing of starch14. Microorganisms are responsible for the production of cellulase and this enzyme can be intracellular or extracellular. Several microorganisms are responsible for cellulase production but very few are capable of degrading the crystalline structure of cellulose. 15.
The process of finishing denim and softening cotton in textile industries is done by cellulases , this enzyme can be used for cleaning in the laundry, for various food processing, in the pulp and paper business, to improve and modify fibre, and in the pharmaceutical industry.16 This indicates that cellulases have applications in various industries and near future there will be a high demand of the enzymes that are stable , active and produced from renewable low-cost raw materials. Thus research to achieve cellulase production using agro waste will prove to be cost-effective and emphasis should be given to the specificity of substrate and enzyme-specific activity. This work was done to optimize various parameters for maximum production of cellulase using potential microbes. It also aimed to study comparatively the isolates showing maximum cellulase-producing ability and to analyze the utilization of agricultural waste as a carbon source by the most potential isolate in the current world production of cellulaes.
Materials and Methods
Isolation of Bacteria
The isolation of Bacteria was done using soil as a sample from the Carpentry shop area located in Mehsana District, Gujarat (C1) and Krishi Vigyan Kendra, Ganpat University, Kherva, Mehsana, Gujarat (C2). The sample was diluted and spread plate method was performed on the CMC agar plate. Incubation of plates was done for 24 hours at 37 °C and 55°C. To observe the zone of hydrolysis, the plate was flooded with congo red (0.1 percent) for 15 minutes and then washed using NaCl (1 M). 17 The diameter of the clear zone created by the colonies on the plate was measured in order to assess the activity of cellulase. The Clear zone diameter of colonies was measured to find the enzyme activity quantitatively. Out of 31 isolates,5 isolates were selected for further process. Additionally, the DNS approach was used to calculate the amount of released reducing sugars.18 The isolate with the highest level of enzyme activity was chosen for improvement.
Table 1: Sampling sites, location and their cellulosic sources.
| Sr. no | Sample Code | Site | Location | Cellulose source |
| 1 | HC | Himmatnagar, Gujarat, India | 23.6°N 72.95°E | Soil from Police housing Corporation construction site |
| 2 | VC | Mansa, Gujarat, India | 23.43°N72.67°E | Vermi Compost soil from farm |
| 3 | C1 | Mehsana, Gujarat, India | 23.6°N 72.4°E | Soil from Carpentry shop area |
| 4 | C2 | Kherva,Mehsana,
Gujarat,India |
23.5443°N 72.443161°E | Soil from Krishi Vigyan Kendra, Ganpat University |
Identification of Bacteria
For Identification of Bacteria various morphological and Biochemical characterizations were done presumptively. These parameters include the morphology of the colony, Catalase test, Indole test, Citrate Utilization test, Urea hydrolysis, Methyl red Test (M-R) test, Voges-Proskauer (V-P) test, Starch hydrolysis, Gelatin hydrolysis, Carbohydrate fermentation test for Glucose, Lactose, Sucrose, Mannitol, Maltose. Bergey’s Manual of Determinative Bacteria was used to compare the results obtained. 19
Medium for Producing Enzyme
The following ingredients were used to create the production medium: peptone 0.75 g, FeSO4 0.01 g, MgSO4 0.5 g, KH2PO4 0.5 g, and glucose 0.5 g (all per litre). In a 100 ml conical flask, 10 ml of medium were added. These flasks underwent a 20-minute autoclave at 121°C. The flasks were further allowed to cool before being inoculated with a bacterial culture that had been cultivated overnight. After that, it was shaken in an incubator for 24 hours at 37°C. To produce a crude extract, the cultured medium was centrifuged at 6000 rpm for 10 minutes.
Enzyme Assay
Cellulase activity was assessed using the Miller technique.18 After that, the reaction mixture was incubated at 37°C for 30 minutes using crude enzyme (0.2 mL) and carboxymethyl cellulose (1.8 mL) produced in sodium phosphate buffer (50 mM-pH 7). To stop the reaction, 3 mL of DNS reagent was added to the mixture. After 5 minutes of boiling the reaction mixture, colour emerged. The optical density (OD) of the samples was measured at 575 nm for additional analysis.
Optimization of process for Maximum Cellulase Production
Incubation Time
The production medium was inoculated with selected colonies, and enzyme activity was monitored every 24 hours.
pH
The pH was adjusted with 1 N HCl and 1 N NaOH to 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0 in several sterile flasks containing broth with the ideal concentration of carbon source and substrate. At 37 °C, the flasks underwent inoculation and incubation. Following the incubation period, filtrate containing cell-free culture was taken for use as an enzyme source in subsequent processes.
Temperature
A chosen bacterial strain that had been cultivated overnight was injected into a production medium at pH 7. The broth was incubated for 24 hours at various temperatures ranging from 35, 40, 45, 50, 55, and 60°C. The cell-free culture filtrate is obtained at the end of the incubation period and used as a source of enzymes.
Carbon Sources
To test their effects, several carbon sources including glucose, lactose, sucrose, fructose, maltose, and CMC were added to the production medium at concentrations ranging from 1 to 5%.
Nitrogen Sources
By substituting urea, yeast extract, and ammonium sulphate for the peptone (0.5%) in the production medium, the effects of various nitrogen sources were studied.
Agro-Based Waste as substrate
Various agricultural waste products, including castor seed waste, wheat bran, and rice husk, were used as the carbon source in the production medium. They were introduced to the growth media while submerged, and after 24 hours, the enzyme activity was assessed.
Result and Discussion
So,a soil sample was used to isolate bacteria that produce cellulase. They were recognised based on their biochemical and physical traits. The isolates showing maximum cellulase activity were found to be Enterobacter cloacae (ISOLATE-C2-A) followed by Pseudomonas aeruginosa (ISOLATE-C1-E) and Bacillus subtilis (ISOLATE-C1-D).
Table 2: Zone of Diameter
| Isolate no. | Code of isolates | Mean Clear zone diameter (ZD mm) | Mean Colony diameter (CD)mm |
| 2 | C1-A | 3±0.10 | 1±0.10 |
| 4 | C1-B | 5±0.15 | 1±0.11 |
| 5 | C1-D | 7±0.05 | 1±0.20 |
| 11 | C1-E | 8±0.10 | 2±0.1 |
| 12 | C2-A | 12±0.15 | 1±0.15 |
Table 3: Cultural and morphological characteristics
| Isolates
|
C1-A
|
C1-B
|
C1-D
|
C1-E
|
C2-A
|
| Size
|
Small | Big | Small | Small | Small |
| Shape
|
Round | Round | rod | Rod | Rod |
| Margin
|
Regular | Irregular | Irregular | Regular | Regular |
| Texture
|
Smooth | Rough | Rough | Smooth | Smooth |
| Consistency
|
Moist | Dry | Moist | Moist | Moist |
| Elevation
|
Low convex | Flat | Convex | Low convex | Low convex |
| Opacity
|
Translucent | Opaque | Translucent | Translucent | Translucent |
| Pigmentation
|
nil | nil | nil | Pale yellow | nil |
| Gram Reaction
|
Positive | Positive | Positive | negative | Negative |
Table 4: Biochemical characterization.
| Biochemical Test | C1-A | C1-B | C1-D | C1-E | C2-A |
| Catalase test | Positive | Negative | Positive | Positive | Positive |
| Indole test | Negative | Negative | Negative | Negative | Negative |
| Citrate Utilization test | Negative | Positive | Positive | Positive | Positive |
| Urea hydrolysis | Negative | Negative | Negative | Negative | Negative |
| Methyl red Test (M-R)test | Negative | Negative | Negative | Negative | Negative |
| Voges-Proskauer (V-P)test | Positive | Negative | Positive | Negative | Positive |
| Starch hydrolysis | Positive | Positive | Positive | Positive | Negative |
| Gelatin hydrolysis | Positive | Positive | Positive | Negative | Positive |
| Glucose | Positive | Positive | Positive | Negative | Positive |
| Lactose | Negative | Positive | Positive | Negative | Negative |
| Sucrose | Positive | Positive | Positive | Negative | Positive |
| Mannitol | Positive | Positive | Positive | Positive | Positive |
| Maltose | Positive | Positive | Positive | Negative | Positive |
Table 5: Enzyme activity by CMC Assay
| Cultural filtrate
(Days) |
Isolate C1-D
(IU) |
Isolate C1-E
(IU) |
Isolate C2-A
(IU) |
| 1 | 61.05±0.12 | 127.66±0.05 | 199.47±0.05 |
| 2 | 77.71±0.32 | 160.97±0.27 | 233.13±0.46 |
| 3 | 116.56±0.24 | 172.07±0.13 | 244.23±0.22 |
| 4 | 105.46±0.15 | 199.82±0.81 | 294.18±0.17 |
| 5 | 66.60±0.31 | 116.56±0.22 | 344.33±0.11 |
| 6 | 122.11±0.02 | 94.36±0.36 | 199.82±0.09 |
| 7 | 49.95±0.10 | 83.26±0.14 | 116.56±0.12 |
Incubation Time
Enzyme activity was checked for all the three isolates by incubating by CMC assay for 7 days. Maximum activity shown by Isolate C1-D, C1-E and C2-A was on days 6th ,3rd and 5th respectively. Amongst these isolates, maximum activity was exhibited by isolate C3 (344.33 IU). [Fig. 1 (a), (b), and (c)]
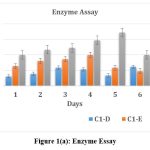 |
Figure 1(a): Enzyme Essay |
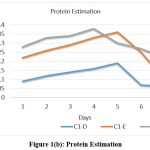 |
Figure 1(b): Protein Estimation Click here to view Figure |
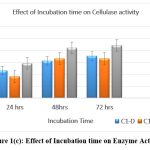 |
Figure 1(c): Effect of Incubation time on Enzyme Activity Click here to view Figure |
Effect of pH
All three isolates were grown on media with pH of different ranges from 5.0 to 11.0. Cellulase activity was maximum observed in a medium with pH 7.0–8.0 for Enterobacter cloacae, Pseudomonas aeruginosa and Bacillus subtilis (Figure 2). The result was also related to the findings of some other researchers for a strain of Bacillus subtilis. 20–22[Figure-2]
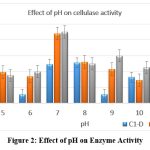 |
Figure 2: Effect of pH on Enzyme Activity Click here to view Figure |
Effect of Incubation Temperature
The production of cellulase peaked at a temperature of 40°C for all three isolates when enzyme activity was measured at various temperatures. [Figure .3]. The secretion of extracellular enzymes was found to be influenced by temperature by causing the cell membrane to change its physical properties. At a temperature of 40°C, these isolates were determined to have the highest enzyme activity23. The results thus obtained had similarity to those of Bakare et al. (2005)24 which suggests that optimum activity for cellulase enzyme obtained from Pseudomonas was at temperatures ranging from 35 to 40°C. Ray et al. 25 observed that the yield of cellulase obtained in a fermentation medium at 45°C was reported to be minimal, while at 40°C, the yield of cellulase was found to be maximum in Bacillus subtilis. Immanuel et al. 7reported the activity of endoglucanase to be maximum in Bacillus, Micrococcus sp. and Cellulomonas , at 40°C and 7 pH [Figure-3]
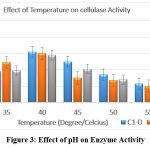 |
Figure 3: Effect of pH on Enzyme Activity. Click here to view Figure |
Effect of Carbon Source
The source of carbon in growth media was replaced by various other carbon sources like lactose, sucrose, fructose, maltose and CMC. Maximum activity was shown by fructose and lactose as substrate after 24 hours of incubation. The best substrate for the formation of cellulase is glycerol, with an efficiency of 28.7% on the weight of additional substrate. 26 When D-xylose is employed as a carbon source, Ishihara et al. showed that purportedly creates cellulase membrane.27Also, it does not show good production of enzyme by selected isolates. Cellulase production was observed at optimum level in presence of the sucrose, maltose and CMC 28. [Figure 4]
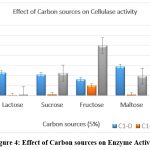 |
Figure 4: Effect of Carbon sources on Enzyme Activity Click here to view Figure |
Effect of Different Concentrations of Carbon Sources
The original concentration of sugar in the growth medium was maintained at 1% which was then replaced by other carbon substrates having 5 percent concentration. Lactose and fructose showed the highest production of cellulase when the medium was supplemented with a 5% carbon source after incubation of 24 h. [Figure 5 a-e]
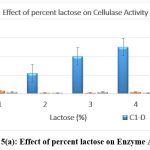 |
Figure 5(a): Effect of percent lactose on Enzyme Activity Click here to view Figure |
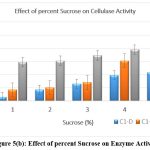 |
Figure 5(b): Effect of percent Sucrose on Enzyme Activity. Click here to view Figure |
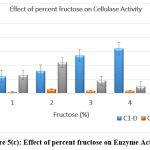 |
Figure 5(c): Effect of percent fructose on Enzyme Activity. Click here to view Figure |
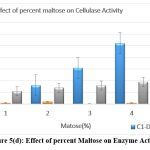 |
Figure 5(d): Effect of percent Maltose on Enzyme Activity Click here to view Figure |
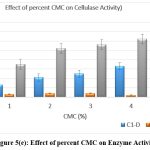 |
Figure 5(d): Effect of percent CMC on Enzyme Activity Click here to view Figure |
Effect of Nitrogen Source
The extracellular production of cellulase depends on the different sources of carbon and nitrogen added to the medium. The role of different nitrogen sources was checked on the growth of isolates by replacing the peptone that was used in the original medium. The various other nitrogen sources used were yeast extract, urea and ammonium sulphate. Amongst these sources, Ammonium sulphate was found to produce maximum cellulase as compared to others. The major element found in the cell proteins contains nitrogen and the uptake of ammonium sulphate salt indicates its direct assimilation in the synthesis of cell proteins.29 [Figure 6, Table-6, Table-7, Table-8].
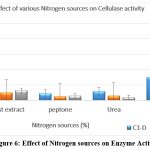 |
Figure 6: Effect of Nitrogen sources on Enzyme Activity Click here to view Figure |
Table 6: Maximum enzyme activity of three isolates considering optimization parameters.
| Maximum Enzyme activity considering optimization parameters | Isolate C1-D | Isolate C1-E | Isolate C2-A |
| Incubation time | 72 hrs
(0.29±0.13 U/mL) |
72 hrs
(0.42±0.31U/mL)
|
72 hrs
(0.44±0.14 U/mL) |
| Optimum pH | 8 (0.51± 0.33U/mL) | 7 (0.88±0.30 U/mL) | 7 (0.90± 0.11U/mL) |
| Optimum Temperature | 40 (0.62± 0.04U/mL) | 40 (0.60±0.21 U/mL) | 40 (0.58±0.02 U/mL) |
| Carbon source | Maltose
(0.58± 0.03U/mL) |
CMC
(0.012±0.13 U/mL) |
Fructose
(1.11±0.21 U/mL) |
| Lactose(%) | 5%(0.65±0.23 U/mL) | 5%(0.03±0.02 U/mL) | 5%(0.02±0.01 U/mL) |
| Sucrose(%) | 5%(0.43± 0.33U/mL) | 5%(0.66± 0.16U/mL) | 5%(0.98± 0.01U/mL) |
| Fructose(%) | 5%(0.35± 0.12U/mL) | 5%(0.03±0.04 U/mL) | 3%(0.22±0.03 U/mL) |
| Maltose(%) | 5%(0.54±0.08 U/mL) | 5%(0.02±0.06 U/mL) | 5%(0.33±0.12 U/mL) |
| CMC(%) | 5%(0.33± 0.04U/mL) | 5%(0.04±0.01 U/mL) | 5%(0.65±0.11 U/mL) |
| Nitrogen source | Ammonium sulphate
(0.30±0.11 U/mL) |
Ammonium sulphate
(0.76±0.05 U/mL) |
Ammonium sulphate
(0.17±0.07 U/mL) |
Table 7: Specific activity of three isolates considering optimization parameters.
| Specific activity considering optimization parameters | Isolate C1-D | Isolate C1-E | Isolate C2-A |
| Incubation time | 72 hrs
(1.52±0.02 U/mg) |
72 hrs
(1.16±0.11U/mg) |
72 hrs
(1.15±0.13 U/mg) |
| Optimum pH | 8 (2.63±0.01 U/mg) | 7 (2.44±0.23 U/mg) | 7 (2.36±0.12 U/mg) |
| Optimum Temperature | 40 (3.26±0.02 U/mg) | 40 (1.66±0.12 U/mg) | 40 (1.52± 0.05U/mg) |
| Carbon source | Maltose
(3.05± 0.23U/mg) |
CMC
(0.03±0.02 U/mg) |
Fructose
(2.92±0.03 U/mg) |
| Lactose(%) | 5%(3.42±0.22 U/mg) | 5%(0.08± 0.23U/mg) | 5%(0.05±0.02 U/mg) |
| Sucrose(%) | 5%(2.26± 0.21U/mg) | 5%(1.83±0.01 U/mg) | 5%(2.57±0.21U/mg) |
| Fructose(%) | 5%(1.84±0.13 U/mg) | 5%(0.08±0.02 U/mg) | 3%(0.57±0.20 U/mg) |
| Maltose(%) | 5%(2.84±0.11 U/mg) | 5%(0.05±0.02 U/mg) | 5%(0.86±0.13 U/mg) |
| CMC(%) | 5%(1.73± 0.02U/mg) | 5%(0.11±0.01 U/mg) | 5%(1.71± 0.01U/mg) |
| Nitrogen source | Ammonium sulphate
(1.57±0.12 U/mg) |
Ammonium sulphate
(2.11±0.23 U/mg) |
Ammonium sulphate
(0.44±0.12U/mg) |
Table 8: Specific activity of three isolates considering optimization parameters.
| Isolate | Incubation time
|
Optimum pH
|
Optimum Temperature
|
Carbon source
|
Nitrogen source
|
Maximum Enzyme Activity | Protein content | Maximum specific activity |
| Isolate C1-D
|
72 hrs | 8 | 40 | Lactose (5%) | Ammonium Sulphate | 0.65±0.23U/ml | 0.19 mg/ml | 3.42±0.22 U/mg |
| Isolate C1-E
|
72 hrs | 7 | 40 | Sucrose (5%) | Ammonium Sulphate | 0.88±0.16U/ml | 0.36 mg/ml | 2.44±0.02 U/mg |
| Isolate C2-A
|
72 hrs | 7 | 40 | Fructose(5%) | Ammonium Sulphate | 1.11±0.98U/ml | 0.38 mg/ml | 2.92± 0.21U/mg |
Effect of Agro-Based Waste Material
Agro waste has been used as a substitute source of carbon in the fermentation medium for the manufacture of cellulase, according to several reports. In contrast to wheat husk and castor seed waste, selected isolate 3 produced the most enzymes, according to the current investigation, when rice husk served as an inducer. [ Table-9, Figure 7]
Table 9: The potential isolate C3’s potential to hydrolyze cellulose from the culture medium and release glucose was used to measure the cellulase activity. The amount of glucose released was measured every 24 hours.
| Period of Fermentation (hours) | Rice husk
IU/mL |
Wheat husk
IU/mL |
Castor seed waste
IU/mL
|
CMC
IU/mL |
| 24 | 0.3 | 0.12 | 0.33 | 0.35 |
| 48 | 0.61 | 0.2 | 0.21 | 0.53 |
| 72 | 0.67 | 0.23 | 0.45 | 0.56 |
| 96 | 1.4 | 0.34 | 0.44 | 0.61 |
| 120 | 1.48 | 1.45 | 0.65 | 1.92 |
| 144 | 1.76 | 1.51 | 0.13 | 1.32 |
| 168 | 0.86 | 1.23 | 0.32 | 0.73 |
| 192 | 0.52 | 0.33 | 0.12 | 0.65 |
| 216 | 0.44 | 0.42 | 0.09 | 0.27 |
| 240 | 0.43 | 0.23 | 0.4 | 0.23 |
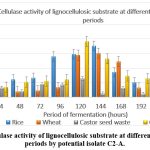 |
Figure 7: Cellulase activity of lignocellulosic substrate at different fermentation periods by potential isolate C2-A. |
Conclusion
In this study, potential cellulase-producing bacteria from soil samples were isolated and identified. Maximum production of cellulase was shown by Enterobacter cloacae followed by Pseudomonas aeruginosa and Bacillus subtilis.
The optimum temperature and pH during cellulase production were found to be 40°C and 7–8. Various carbon and nitrogen sources were used and amongst them the best result was given by lactose and ammonium sulphate as compared to others. As a result, this research has assisted in identifying numerous optimized criteria for the microorganism’s maximal cellulase production. The production of cellulase was carried out after optimization of various parameters at 40°C, pH 7 for 48 hours in shaking incubator at 120 rpm. It is found that in comparison to fungi, bacteria show a higher growth rate with good potential to produce cellulase. However, using bacteria to produce cellulase is not a common practice.
Reports suggested the presence of cellulolytic activity in some bacterial genera like Cellovibrio, Sporocytophaga, Cellulomonas, Pseudomonas, spp. 9, Bacillus, and Micrococcus 7. This research suggested that several parameters are responsible for the production of the enzyme in microorganisms and control the overall process of optimization. These parameters include pH, inoculum time, temperature, carbon and nitrogen sources, etc. which play a vital role in the total yield of cellulase. Enzyme activity and specific enzyme activity for each isolate were calculated and thus the potential of these isolates was compared. The ability of these bacteria to produce cellulase in the presence of different carbon sources can be beneficial in optimizing the production process for each isolate.
Additional research on the purification and use of cellulase in several commercial domains is ongoing. The detergent, food, and pharmaceutical industries can all benefit from the utilisation of the refined cellulase. Cellulase enzymes’ high activity and stability at high temperatures and neutral to alkaline pH will be useful in a variety of industrial and biotechnological applications.
Conflict of Interest
There is no conflict of interest
Funding Sources
there is no funding sources.
References
- Tomme P, Warren R.A, Gilkes N.R. Cellulose hydrolysis by bacteria and fungi. Advances in microbial physiology. 1995 Jan 1; 37:1-81.
CrossRef - Jarvis M. Cellulose stacks up Nature. 2003 Dec 11;426(6967):611-2.
CrossRef - Zhang Y.H, Lynd L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: non complexed cellulase systems. Biotechnology and bioengineering.2004 Dec 30;88(7):797-824.
CrossRef - Bahkali A.H. Influence of various carbohydrates on xylanase production in Verticillium tricorpus. Bioresource Technology.1996 Sep 1;57(3):265-8.
CrossRef - Magnelli P, Forchiassin F. Regulation of the cellulase complex production by Saccobolus saccoboloides: induction and repression by carbohydrates. Mycologia. 1999 Mar 1;91(2):359-64.
CrossRef - Shin C.S, Lee J.P, Lee J.S, Park S.C. Enzyme production of Trichoderma reesei Rut C-30 on various lignocellulosic substrates. In Twenty-First Symposium on Biotechnology for Fuels and Chemicals: Proceedings of the Twenty-First Symposium on Biotechnology for Fuels and Chemicals Held May 2–6, 1999, in Fort Collins, Colorado 2000 (pp. 237-245). Humana Press.
CrossRef - Immanuel G, Dhanusha R, Prema P, Palavesam A.J. Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. International Journal of Environmental Science & Technology.2006 Dec;3:25-34.
CrossRef - Bhat M.K. Cellulases and related enzymes in biotechnology. Biotechnology advances. 2000 Aug 1;18(5):355-83.
CrossRef - KATSUMI N, KUMPEI K. Isolation and identification of crystalline cellulose hydrolyzing bacterium and its enzymatic properties. Journal of fermentation technology. 1982;60(4):343-8.
- Lee Y.J, Kim B.K, Lee B.H, Jo K.I, Lee N.K, Chung C.H, Lee Y.C, Lee J.W. Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresource technology. 2008 Jan 1;99(2):378-86.
CrossRef - Saha S, Roy R.N, Sen S.K, Ray A.K. Characterization of cellulase‐producing bacteria from the digestive tract of tilapia, Oreochromis mossambica (Peters) and grass carp, Ctenopharyngodon idella (Valenciennes). Aquaculture Research. 2006 Mar;37(4):380-8.
CrossRef - Nishida Y, Suzuki K.I, Kumagai Y, Tanaka H, Inoue A, Ojima T. Isolation and primary structure of a cellulase from the Japanese sea urchin Strongy locentrotus nudus. Biochimie. 2007 Aug 1;89(8):1002-11.
CrossRef - Pranner J. Environmental microbiology and waste utilization. G/AMV Proceedings. 1979:67-9.
- Camassola M, Dillon A.J. Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid‐state fermentation. Journal of Applied Microbiology. 2007 Dec 1;103(6):2196-204.
CrossRef - Koomnok C. Selection of cellulase producing thermophilic fungi. In Proceedings of the 31st Congress on Science and Technology of Thailand of Technology 2005 Oct. Suranaree University.
- Cherry J.R, Fidantsef A.L. Directed evolution of industrial enzymes: an update. Current opinion in biotechnology. 2003 Aug 1;14(4):438-43.
CrossRef - Apun K, Jong B.C, Salleh M.A. Screening and isolation of a cellulolytic and amylolytic Bacillus from sago pith waste. The Journal of general and applied microbiology. 2000;46(5):263-7.
CrossRef - Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry. 1959 Mar 1;31(3):426-8.
CrossRef - .Buchanan R.E, Gibbons N.E. Bergey’s of determinative bacteriology. America: United States of America. 1974:529-63.
- Chantawannakul P, Oncharoen A, Klanbut K, Chukeatirote E, Lumyong S. Characterization of proteases of Bacillus subtilis strain 38 isolated from traditionally fermented soybean in Northern Thailand. Science Asia. 2002 Sep;28(4):241-5.
CrossRef - Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NA. Optimization of surfactin production by Bacillus subtilis isolate BS5. Applied Biochemistry and Biotechnology. 2008 Sep;150:305-25.
CrossRef - Wu M, Zhang L, Li D, Wang Y, Zhang Z, Mao Z. Conditions Study of Cellulase and Acid Protease Production during the Process of Solid-state Fermentation of Flaxseed Meal. In2008 Providence, Rhode Island, June 29–July 2, 2008 2008 (p. 1). American Society of Agricultural and Biological Engineers.
- Jansová E, Schwarzová Z, Chaloupka J. Sporulation and synthesis of extracellular proteinases in Bacillus subtilis are more temperature-sensitive than growth. Folia microbiologica. 1993 Feb;38:22-4.
CrossRef - Bakare M.K, Adewale I.O, Ajayi A, Shonukan O.O. Purification and characterization of cellulase from the wild-type and two improved mutants of Pseudomonas fluorescens. African Journal of Biotechnology. 2005;4(9).
- Ray A.K, Bairagi A, Ghosh K.S, Sen S.K. Optimization of fermentation conditions for cellulase production by Bacillus subtilis CY5 and Bacillus circulans TP3 isolated from fish gut. Acta Ichthyologica et Piscatoria. 2007 Jun 15;37(1):47-53.
CrossRef - Ishihara M, Matsunaga M, Hayashi N, Tišler V. Utilization of D-xylose as carbon source for production of bacterial cellulose. Enzyme and Microbial Technology. 2002 Dec 2;31(7):986-91.
CrossRef - Oikawa T, Morino T, Ameyama M. Production of cellulose from D-arabitol by Acetobacter xylinum KU-1. Bioscience, biotechnology, and biochemistry. 1995 Jan 1;59(8):1564-5.
CrossRef - Ramana KV, Tomar A, Singh L. Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World Journal of Microbiology and Biotechnology. 2000 Apr;16:245-8.
CrossRef - Mandels M. Microbial sources of cellulase. In Biotechnol. Bioeng. Symp. 1975 (Vol. 5, pp. 81-105).

This work is licensed under a Creative Commons Attribution 4.0 International License.





