How to Cite | Publication History | PlumX Article Matrix
Mitali Singh1* , Vijay Sharma2
, Vijay Sharma2 , Alankar Shrivastav2
, Alankar Shrivastav2 , Pawan Singh2
, Pawan Singh2 and Navneet Verma2
and Navneet Verma2
1Sahu Onkar Saran School of Pharmacy IFTM University Moradabad, Uttar Pradesh, India.
2Pharmacy Academy, Iftm University, Moradabad, Uttar Pradesh, India.
Corresponding Author E-mail: vijaysrampur@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3165
ABSTRACT: The goal of nanotechnology is to develop new nanoscale components by designing, fabricating, and using materials that are atomically, molecularly, and macromolecular organized. For several decades, there has been an abundance of scientific curiosity in the administration of medicines using particle delivery systems as carriers for small and big molecules. Particulate systems, such as nanoparticles, have been utilized as a physical method to modify and improve the pharmacokinetic and pharmacodynamics aspects of numerous pharmaceutical molecules. A typical nanoparticle is between 1 and 100 nm in size and has one or more dimensions. Nanoparticles are usually categorized as inorganic, organic, or carbon-based particles according to their superior characteristics in comparison to larger sizes of the corresponding materials. They have been utilized in vivo to protect the drug entity in the systemic circulation, limiting drug distribution to the targeted areas, and to transport the drug at a controlled and sustained rate to its site of action. The most innovative and promising medication delivery technique at the moment is nanoparticle technology. This methodical research examines the categorization, characteristics, techniques, characterizations, and applications of nanoparticles in the delivery of drug molecules.
KEYWORDS: Applications; Characterization; Drug delivery; Nanotechnology; Nanoparticles; Properties
Download this article as:| Copy the following to cite this article: Singh M, Sharma V, Shrivastav A, Singh P, Verma N. Nanotechnology for Novel Drug Delivery: A Systematic Review of Classification, Preparation, Characterization, and Applications of Nanoparticles in Drug Delivery. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Singh M, Sharma V, Shrivastav A, Singh P, Verma N. Nanotechnology for Novel Drug Delivery: A Systematic Review of Classification, Preparation, Characterization, and Applications of Nanoparticles in Drug Delivery. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/3QVvIit |
Introduction
Nanotechnology is a comprehensive branch of technology that entails the creation and use of numerically calculated-scale materials, systems, or devices. Nanotechnology has been employed in nearly every aspect of everyday life as a result of its unique and inventive applications in a wide range of areas. The twenty-first century, also referred to as a “nano-century,” has been marked by nanotechnology as one of the most significant scientific efforts, with nanotechnology having an impact on many aspects of daily life. It is developing a range of goods with uses in many fields, including imaging, diagnostics, and the administration of medications.1 Over the past ten years, the prefix “nano” has become more and more applicable in a variety of scientific fields. A large public, including non-experts, is now familiar with a variety of new nano-related terminology that has appeared often in scientific articles and popular media. Nanoscience, nanotechnology, nanomaterials, and nanochemistry are some of the words that fall under this category. The origin of the word “preface” may be traced back to the Latin word “nanus,” which literally translates to “extremely little.” Within the framework of the agreement governing the International System of Units, it is used to denote a factor of reduction that is 109 times greater. Because of this, the nanosized cosmos commonly has dimensions that are measured in nanometers, and it comprises systems that are bigger than molecular dimensions but smaller than macroscopic dimensions (which typically have values that fall between 1 nm and 100 nm).2 There hasn’t been much work done in the area of nanotechnology research. It requires making economical use of available resources. Atoms and molecules act differently at this level, and as a result, this level may be utilized for a wide range of absorption applications. Recent years have seen a proliferation of research in several product domains pertaining to nanotechnology. It makes the production of substances possible, which is very useful in the medical field because there are often limitations placed on the predictability of treatment procedures. It is important to avoid thinking of nanotechnology as a singular strategy with few potential applications. Although it is sometimes referred to as “the little science,” nanotechnology is employed for much more than only the creation of things and structures that are extremely small. Nanoscale features are regularly incorporated into large surfaces as well as bulk materials.3 It is possible for pharmaceutical nanoparticles, which are solid drug carriers that are less than 100 nm in size, to be biodegradable. However, it is also possible for them not to be biodegradable. The term “nanoparticle” can refer to either nanospheres or nanocapsules depending on the context. Nanospheres are a grid structure in which the medication is evenly spread, whereas nanocapsules are a system in which the medication is encapsulated by a unique polymeric film. This in-depth study has a strong emphasis on the categorization of nanoparticles, as well as their processes of production and characterization, as well as the uses of these particles.4, 5 The nanomaterial graphene is widely recognised. Graphite, which is the most stable kind of carbon, is used in industry as both a lubricant and as the ‘lead’ in pencils. Graphite is a layered product composed of carbon atom sheets which form hexagonal patterns replicating benzene rings in each layer. Graphene is the name given to a monolayer of graphite.6
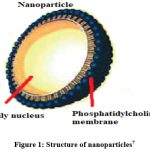 |
Figure 1: Structure of nanoparticles7
|
Define nanoparticles and nanomaterials by other organizations
Table 1. Many organizations clarify nanoparticles along with nanomaterials as follows: 8, 9
|
Organization |
Nanoparticles |
Nanomaterials |
|
“American Society of Testing and Materials |
A nanoscale particle has a length between 1-100 nm across at least two locations. |
– |
|
International Organization for Standardization |
A particle with 1-100 nm in diameter. |
– |
|
National Institute of Occupational Safety and Health |
A particle (1 and 100 nm) or a filament with a diameter of 1 to 100 nm. |
– |
|
Scientific Committee on Consumer Products |
A minimum of one side is nanoscale in size. |
Materials with at least one nanoscale side or internal structure |
|
British Standards Institution |
All of the areas and dimensions are on the nanoscale. |
Materials having one side or internal structure is in the nanoscale |
|
Bundesanstalt für Arbeitsschutz und Arbeitsmedizin |
All the zones or diameters have a nanoscale range. |
Materials that are created from a nanostructure or a nanosubstance” |
Table 2: Merits and Demerits of Nanoparticles.
|
“Merits |
Demerits |
|
* After intramuscular administration, nanoparticle particle size and surface characteristics could be readily improved to provide both active as well as passive medicine delivery. |
* If taken in excess, nanoparticles might harm the body’s immune system. 13, 14 |
|
* Controlling and sustaining medication release throughout transportation and localization is critical to obtaining high pharmacological therapeutic effectiveness with minimal adverse effects. |
* Fine particle inhalation can cause illnesses such as silicosis, cancer, and emphysema.15 |
|
* Targeting ligands can be included in particles to deliver site-specific targeting, which is magnetic direction can be used.10 |
* Nanoparticles are not absorbed through the skin because they irritate the skin’s surface.16 |
|
* The system can be applied for oral, integration-ocular, parenteral, and nasal dosing.11 |
|
|
* Nanoparticles can improve medicine delivery to small locations within the body.12 |
|
Classification
Organic, inorganic, as well as carbon-based nanoparticles are among the three types.
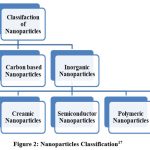 |
Figure 2: Nanoparticles Classification17
|
Organic NPs
Organic nanoparticles can have several forms, some of the most well-known of which include micelles, dendrimers, ferritin, and liposomes. Some nanoparticles, such as micelles and liposomes, have hollow cores that are referred to as thin film-capsules. These thin film-capsules are sensitive to electromagnetic and thermal radiation. These nanoparticles are frequently utilized in the pharmaceutical industry as a result of the fact that they are inexpensive, that they may be transported into particular regions of the body, and that they are employed in medicine delivery systems. This tactic is sometimes referred to as “target drug delivery,” which is a word used to describe it. Examples: liposomes, dendrimers, micelles, etc.18
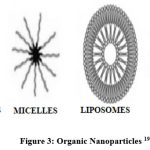 |
Figure 3: Organic Nanoparticles19
|
Inorganic NPs
These are carbon-free nanoparticles. Examples: Metal and metal oxide.
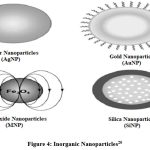 |
Figure 4: Inorganic Nanoparticles20
|
Metal NPs
“Aluminum (Al), cadmium (Cd), cobalt (Co), copper (Cu), and zinc (Zn) are the metals that are most frequently employed in the manufacturing of nanoparticles. Metal nanoparticles can be produced using chemical reducing agents by lowering the concentration of metal-ion precursors in the solution. These can take in small particles and have a high-energy surface. Researchers have employed these nanoparticles for biomolecule identification, picture processing, environmental analysis, and other purposes. For example, gold nanoparticles have been used to shield the object of study prior to SEM analysis. This was commonly done to increase electrical flow, enabling us to obtain better SEM pictures. Nanoparticles of metal have been used in a variety of scientific fields of research because of their outstanding optical properties.
Ceramic NPs
These particles are inorganic solids comprised of carbide particles, carbonates, and phosphates that are oxides of iron, created via heating and cooling. Polycrystalline, dense, amorphous polycrystalline, dense, porous, and hollow variants are of great interest to researchers. By altering their chemical composition, they may be manufactured in drug delivery systems, which is particularly beneficial in the treatment of bacterial infections, glaucoma, and many types of malignancies.
Semiconductor NPs
The periodic table contains semiconductor nanoparticles in groups (II–VI, III–V, or IV–VI). When tuned, they exhibit a range of features and have broadband gaps. Applications for them include photocatalysis, electronics, photonics, and water splitting. Examples of nanoparticles constructed of semiconductors from groups III–V include GaN, GaP, INP, and InAs. A few examples of semiconductor nanoparticles from groups II to VI include ZnO, ZnS, CdS, CdSe, and CdTe.21
Polymeric NPs
These particles are mostly organic-based nanoparticles, and the scientific community refers to them collectively as “polymer particulate” (PNP). Depending on how much preparation has been done, these are either nanospheres or nanocapsules. In contrast to the latter, which are adsorbed at the sphere’s outer edge, the former are matrix molecules with a generally solid overall mass. In the latter scenario, the particle entirely encloses the solid mass. Due to its simplicity in functionalization. Polymer NPs provides Controlled release, drug molecule protection, the potential to combine therapy with imaging, selective targeting, and many other advantages. They can be used for both diagnosing problems and administering medications. Medication distribution by polymeric nanoparticles is non-toxic and biodegradable.
Lipid-based NPs
These particles are spherical and in range from 10 to 100 nm. They consist of a solid lipid-based core and a matrix of soluble lipophilic molecules that are emulsified and stabilized by surfactants. In biomedicine, they serve as RNA release mechanisms in cancer treatment as well as drug transporters and delivery systems.
Carbon-based NPs
CNTs and fullerenes are the two major components of carbon nanostructures. Carbon nanotubes are folded graphene sheets that are 100 times stronger than steel and it is used for structural reinforcement. Heat is dispersed throughout the length of CNTs but not evenly throughout the tube. A carbon allotrope made up of sixty or more carbon atoms is the material known as Buck. The carbon atoms that make up these structures are arranged in pentagonal and hexagonal configurations.22 Because of their electrical conductivity, high strength, and strong electron affinity, they have been used in trade. Because the rolled sheets can have one, or more walls, they are referred to as single-walled, double-walled, or multi-walled carbon nanotubes. They are frequently created by coating metal particles with carbon precursors, particularly molecular carbons that have been evaporated from graphite using an electric arc. They were just recently produced utilizing the CVD method (chemical vapor deposition). These materials are used for applications, including fillers, effective gas adsorbents, and support media for various inorganic and organic catalysts.23, 24
 |
Figure 5: Carbon-based Nanoparticle25
|
Properties: Table 3: shows the physiochemical characteristics of nanoparticles.
|
“Nanoparticles types |
Properties |
References |
|
Metal-based nanoparticles |
||
|
Aluminum |
Highly reactive, sensitive, and wide surface area |
26 |
|
Iron |
Unstable and unsteady, air (oxygen) and water are susceptible |
27 |
|
Silver |
Absorbs remains stable, antibacterial, and disinfecting |
28 |
|
Gold |
Reactive to visible light, interactive |
29 |
|
Cobalt |
An unstable, conductive, poisonous substance that absorbs |
30 |
|
Cadmium |
Semiconductor |
31 |
|
Lead |
Strong toxicity, strong reactivity, and high stability |
32 |
|
Copper |
Ductile, highly combustible solids with high thermal and electrical conductivity |
33 |
|
Zinc |
UV filtering, antibacterial, anticorrosive, and antifungal properties |
34 |
|
Carbon-based nanoparticles |
||
|
Fullerenes |
Semiconductors, conductors, and superconductors are all considered safe and inert materials. |
35 |
|
Graphene |
Extraordinary strength, thermal conductivity, and electrical conductivity |
36 |
|
Carbon Nano Tubes (CNT) |
Strong electrical and thermal insulation, a high tensile strength, and a capacity to remain flexible and elastic |
37 |
|
Carbon Nanofiber |
Thermal, frequency shielding, and mechanical qualities that are extraordinary |
38 |
|
Carbon Black |
Surface area; excellent durability and electrical conductivity; resistance to sunlight |
39 |
|
Metal oxide-based nanoparticles |
||
|
Titanium oxide |
Magnetic surface area reduces bacterial growth |
40 |
|
Iron oxide |
Reactive and volatile |
41 |
|
Magnetite |
Highly reactive magnetic |
42 |
|
Silicon dioxide |
Durable, less noxious, and capable of functionalizing a wide range of compounds |
43 |
|
Zinc oxide |
UV filtering, antibacterial, anticorrosive, and antifungal characteristics |
44 |
|
Cerium oxide |
Antioxidants with modest potential for a decrease |
45 |
Physical Properties
The visual, mechanical, and electrical characteristics of nanoparticles are crucial to their use. Color, the capacity to both absorb and reflect light, the capacity to both absorb and reflect ultraviolet radiation, as well as the qualities of being elastic, ductile, tensile, and flexible, are a few examples of these traits. In addition to their magnetic and electrical properties, which include conductivity, semi conductivity, and resistivity, they also include hydrophilicity, hydrophobicity, suspension, and settling traits. As a result, nanoparticles are now used in contemporary electronics for applications like thermal conductivity.
Chemical Properties
These properties include reactivity, stability, and susceptibility to various variables. They are useful for biological and environmental applications because of their antibacterial, antifungal, disinfecting, and toxicological characteristics. They are also oxidative, reducible, flammable, anti-corrosive, corrosive, and anti-corrosive.46
Methods of nanoparticle preparation:
Here are a few techniques for creating nanoparticles.
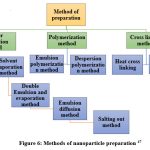 |
Figure 6: Methods of nanoparticle preparation 47
|
Cross-Linking Techniques
In this technique, amphiphilic macromolecules, proteins, and polysaccharides, which have a high affinity for both aqueous and lipid solvents, are used to create the nanoparticles. Amphiphilic molecules are first aggregated, then further stability is added by heat denaturation or chemical cross-linking. Bovine serum albumin, or protein aqueous solutions in oil are emulsified using high-frequency sonication. Heat cross-linking occurs when the resultant water-only emulsion is added to warmed oil. In order to denature, agglomerate, and evaporate water, the protein solution in hot oil is stirred for a while. Before being centrifuged to separate the produced particles, they were washed with a natural solvent to get rid of any remaining oil. For materials that are susceptible to heat, chemical cross-linking is applied.
“Cross-linking can be done by two methods:”
Table 4: Heat and chemical cross-linking method47
|
“Steps |
Heat cross-linking |
Chemical cross-linking |
|
Step 1. |
Aq. solution + oily solution |
Preparation of aq. Solution of polymer |
|
Step 2. |
W/O emulsion |
The ethyl acetate solution in chloroform is emulsified, and cross-linking is accomplished with a cross-linking agent (such as 3% glutaraldehyde). |
|
Step 3. |
W/O emulsion is now poured in warmed oil (100) |
Stir it for several hours |
|
Steps 4. |
Aqueous protein degradation and aggregation, as well as water evaporation |
Formulation of Nanospheres |
|
Steps 5. |
The formulation of proteinaceous particles |
Washed with toluene or water and freeze-dried it. |
|
Step 6. |
Centrifugation is used to capture oil residues after washing with an organic solvent.” |
|
Nanoparticles prepared by the polymerization method
Emulsion polymerization
In order to generate nanoparticles, monomers undergo polymerization in an aqueous solution. Adsorption or dissolution are the two methods that are used to incorporate drugs into nanoparticles. By ultracentrifuging the nanoparticle solution, the stabilizers, and surfactants that were used in the polymerization process may be removed. The nanoparticles can then be re-suspended in a medium that is isotonic and does not include any surfactants. It has been utilized in the production of nanoparticles made of polybutylcyanoacrylate or poly (alkyl cyanoacrylate).48-51
Dispersion polymerization
A homogeneous system is produced when monomers, initiators, and stabilizing agents are dissolved in an organic solvent and form polymer particles. The monomers and initiators are utilized as reaction media in this approach and are readily dissolved in a non-solvent. An aqueous monomer solution instantly undergoes nucleation. As a result, neither a stabilizer nor a surfactant is required. The same strategy as the polymerization of emulsion by high-energy radiation is used for initiation. The process of polymerization begins with the addition of a catalyst and continues through the nucleation and development phases.52-53
Table 5: Steps involved in the Emulsion polymerization method and Dispersion polymerization method47
|
“Steps |
Emulsion polymerization |
Dispersion polymerization |
|
Step 1. |
Polymer is added to the drug solution along with the drug |
Addition of monomers and stabilizers in a solvent from a homogenous solution |
|
Step 2. |
The Addition of a surfactant causes micelle formulation |
Polymerization is initiated by various mechanisms |
|
Step 3. |
Polymerization will occur after initiators are added |
The process of Nucleation occurs |
|
Step 4. |
After polymerization, the formulation of solid particles occurs |
|
Nanoparticles prepared by the polymer precipitation method 63
Emulsion-Solvent Evaporation
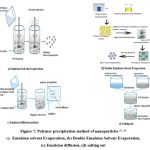 |
Figure 7: Polymer precipitation method of nanoparticles 63, 66
|
This technique produces a large number of nanoparticles. There are two phases to this process. The first step is the emulsification of the polymeric solution in the aqueous phase. In the second stage, the polymer solution evaporates, and nano-spheres are created by causing polymer precipitation. The nanoparticles are gathered by ultracentrifugation, and any free drugs or leftovers are removed before they are washed with distilled water and lyophilized for storage.54 High-pressure emulsification and solvent evaporation are other names for this process.55 To get rid of the organic solvents, this procedure has to be homogenized under high pressure and stirred all around.56 Size, viscosity and the quantity of the dispersion agent of the organic phases, and their stirring rate may all be controlled.57 This method, however, is restricted to the use of fat-soluble medications due to growth concerns. The polymers used in this approach are PLA and Poly (hydroxybutyrate) (PHB) 58, Poly (caprolactone) (PCL) 59, [Poly (lactic-co-glycolic acid) PLGA 60, cellulose acetate phthalate60, and ethyl cellulose62.
Double Emulsion and Evaporation
This method’s main flaw is the ineffective trapping of hydrophilic medications. As a result, the double emulsion method—in which aqueous drug solutions are added to organic polymer solutions while violently spinning to create emulsions—is employed to encapsulate hydrophilic medicines. To create a mixed emulsion (w/o/w), this w/o emulsion is added to another continuous phase and continuously stirred. 63Next, the solvent is evaporated, and high-speed centrifugation can be used to separate the nanoparticles. Before lyophilization, the generated nanoparticles need to be cleaned. The amount of hydrophilic medication incorporated, the amount of polymer, the volume of the aqueous phase, and the concentration of the stabilizer are the variables used in this technique. These elements also affect how nanoparticles are characterized.63
Emulsion Diffusion
Emulsion diffusion is a common technique for producing nanoscale particles. The initial thermodynamic equilibrium of both saturated solutions is guaranteed when the encapsulating polymer is dissolved in an organic solvent that is moderately miscible with water, such as propylene carbonate or benzyl alcohol. The next step is to emulsify the polymer-water-saturated solvent phase in an aqueous solution containing a stabilizer. This causes the solvent to diffuse to the outer phase and, depending on the oil-to-polymer ratio, causes the production of nanospheres or nanocapsules. In the end, the solvent is removed either by evaporation, and the method used depends on the boiling point of the solvent. This approach has several advantages, including high encapsulation efficiency (typically 70 percent), simplicity, batch-to-batch repeatability, and a restricted size distribution. Additionally, this method has a low requirement for homogenization. This approach has a number of problems, including the need to remove a lot of water from the suspension and worse encapsulation effectiveness during emulsification because water-soluble drug leakage occurs in the saturated-aqueous outer phase.67A few examples of drug-loaded nanoparticles that may be created using this method are doxorubicin-loaded PLGA NPs70, cyclosporine (CY-A-); filled sodium glycolate NPs68, and mesotetra (hydroxyphenyl) porphyrin-loaded PLGA (p-THPP) NPs.69
Salting-out
A salting-out approach modifies the emulsification/solvent dispersion process by combining the medicines and polymer in a solvent. Acetone is frequently used because it can be readily removed and is water-soluble. Then, salting-out agents and colloidal stabilizers are added, and the mixture is emulsified into an aqueous gel. It is necessary to dilute the o/w emulsion with enough water to allow acetone to enter the continuous phase and create nanospheres.71,72
Table 6: Literature survey of different preparation methods of nanoparticles in Drug Delivery.
|
Author name |
Year |
Drug |
Polymer |
Method |
Result |
Ref. |
||
|
Leena Peltonen |
2002 |
Sodium cromoglycate |
Polylactic acid |
Nanoprecipitation method |
By using a modified nanoprecipitation process, one can drastically improve the characteristics of PLA nanoparticles by changing the cosolvent in the inner phase. |
73 |
||
|
Deepak Sharma |
2014 |
Lorazepam |
PLGA, Poloxamer |
Emulsion Solvent evaporation method |
Lorazepam-loaded polymeric nanoparticles using PLGA as the release-regulating polymer demonstrated promising results when optimized utilizing a 4-factor, 2-level Box-Behnken design. |
74 |
||
|
Sarvesh Bohrey |
2016 |
Diazepam |
PLGA |
Emulsion Solvent evaporation method |
We may conclude that several preparation variables control the creation of drug-loaded nanoparticles using the emulsion solvent evaporation method. |
75 |
||
|
Umesh D. Laddha |
2014 |
Pioglitazone |
Poloxamer 407 |
single emulsion solvent evaporation method |
Using 5 Poloxamer 407 and HPMC K4M, we created PPAR-agonist PLGA NPs loaded in situ gel used for the treatment of dry eye illness. |
76 |
||
|
Nazimuddin Chishti |
2019 |
Docetaxel |
PLGA, poloxamer 188 |
single emulsification-solvent evaporation |
As part of QbD investigations, we investigated formulation and process factors on NP CQAs using experimental design methods Placket-Burmann and Box-Behnken. |
77 |
||
|
Rajesh Kesarla |
2014 |
Moxifloxacin |
Poloxamer 407, Eudragit RL 100 |
Emulsion solvent evaporation method |
Moxifloxacin formulation was found to be liquid having valid pH and find gel in the presence of monovalent or divalent cations. |
78 |
||
|
Surendranath Betala |
2018 |
Carvedilol |
HPMC, Gelatin, Chitosen |
Nanoprecipitation method |
Among the several nanoparticulate formulations created using the nanoprecipitation process, formulation NP 2 containing chitosan in a 1:1 drug: polymer ratio was selected. |
79 |
||
|
Bader Mubarak Aljaeid |
2016 |
Miconazole |
Poloxamer 188 |
Hot emulsification/ultrasonication method. |
Miconazole formulation in oral solid lipid nanoparticles, a new drug delivery technology, increased bioavailability compared to commercial capsules and was more effective in the treatment of candidiasis. |
80 |
||
|
A.A. Kharia |
2012 |
Acyclovir |
(Bovine serum albumin, chitosan, and gelatin) |
Nanoprecipitation methods method |
Nanoparticle formulations created by nanoprecipitation method. |
81
|
||
Evaluation of Nanoparticles
Zeta potential
“It is usual practice to use a nanoparticle’s Zeta potential to ascertain the surface charge properties of the particle. The structure of the particles themselves as well as the medium in which they are dispersed have an impact on this depiction of the electrical potential of the particles. Nanoparticles must have zeta potentials greater than 30 mV in order to maintain stability while in suspension because the surface charge prevents particle agglomeration.82
Particle Shape
The Nano suspension is lyophilized to produce solid particles before being evaluated and subjected to SEM analysis.83
Particle size
The most crucial factors to consider while studying nanoparticle systems are particle diameters and size distributions. They assess the system’s capabilities for targeting, toxicity, biological destination, and in vivo dispersion. They may also affect the loading, release, and stability of nanoparticles, in addition to other things. The technique that has been shown to be the most effective and dependable for figuring out the sizes of individual particles is photon-correlation spectroscopy, commonly referred to as dynamic light scattering. The data produced from photon-correlation spectroscopy are often validated using scanning or transmission electron microscopy (SEM or TEM).84
Drug Entrapment Efficiency
The continuous medium was separated from the NPs by ultracentrifuging them at a speed of 10,000 rpm for 30 minutes at a temperature of 50 °C. Then, phosphate-buffered saline, which has a pH of 7.4, was added to the mixture after the supernatant solution was discarded. To guarantee that any drug molecules that had eluded capture were destroyed, the technique was repeated twice more. By deducting the entire amount of drug used to create the NPs from the total amount of drug present in the aqueous medium, the amount of drug that was trapped in the nanoparticles was determined. As a result, the researchers were able to calculate how much medication was present inside each nanoparticle.85
Drug entrapment efficiency (%) = amount of drug released ÷ amount of drug used to manufacture the nanoparticles × 100
In-vitro Study
A USP Type II dissolving device with a 50-rpm rotation speed is used for the procedure. The preparation has to be maintained at 370.20 °C and immersed in 900 ccs of phosphate buffer solution in a vessel. A constant volume was maintained by taking 5 ml of the medium at regular intervals and replacing it in the tank with the same quantity of dissolving media. A UV spectrophotometer is used to evaluate the removed samples.86
Stability
By storing the formulation at 4°C, 1°C, and 30°C, 2°C for 90 days, the stability of the generated nanoparticles was examined. Following some time, such as 0, 1, 2, and 3 months, the samples were analyzed again to see whether there had been any changes to their physical characteristics, drug content, or drug release rate.87
Structure and Crystallinity
A number of different approaches might be utilized to ascertain the structure as well as the crystallinity of a substance. The method that is utilized the most frequently for determining crystallinity and structure is known as X-ray diffraction. The production of X-rays begins when a stream of high-energy electrons collides with a target made of metal. This is how X-ray imaging gets started. The X-ray source cannot be observed due to the presence of a filter. Low-energy rays, on the other hand, are able to penetrate a patient and settle on a piece of photographic film, in contrast to high-energy rays. X-rays are capable of penetrating solids, gases, and liquids alike. The strength, rarity, and wavelength of the X-ray photons all play a role in determining where the point of penetration will be.
The yield of Nanoparticles
It is possible to calculate the yield of particles by comparing the total weight of the nanoparticles that were generated to the total weight of the drug and the copolymer combined. “
% Yield = (Amount of Nanoparticle ÷Amount of Drug + Polymer) × 100”××100
Drug Content
The standard calibration curve was then constructed using a UV spectrophotometer to quantify the amount of medication in the supernatant. The total amount required to produce the nanoparticles is then reduced by the amount of medication present in the supernatant. The % of drug entrapment as determined by.”88
% Drug Entrapment = (total drug conc.- total supernatant conc.) /total drug conc. × 100
Applications of Nanoparticles in Novel Drug Delivery
 |
Figure 8: “Applications of nanoparticles in various drug delivery systems”
|
In Tumor Targeting
This article explains how nanoparticles are used to target tumors.
“NPs will be provided a concentrated dosage of medicine close to tumor targets because of increased penetration and retention, or active targeting by ligands.
Due to their ability to restrict medication distribution to the intended organ, nanoparticles can reduce drug exposure to healthy tissues.”
Standard nanoscale particles are expected to cause significant cytotoxicity against Kuepfer cells when used as transporters in chemotherapy treatment. This will result in a reduction in the number of Kuepfer cells and, as a natural consequence, a diminished therapeutic impact and liver absorption when administered at intervals of less than two weeks. The bone marrow is a vital but unwanted action for the majority of anticancer drugs since therapy with such carriers may increase myelosuppression. Conventional nanoparticles have the ability to target the bone marrow, which is a key but undesirable site of action. As a consequence of this, the only malignancies that can benefit from utilizing conventional nanoparticles to boost the efficiency of anticancer therapy are the ones that occur at the level of organs that contain a high concentration of MPS. In addition, if nanoparticles are removed rapidly after intravenous infusion, it is hard to target anticancer drug-loaded nanoparticles to diverse places within a tumor.23
In brain drug delivery
The blood-brain barrier, often known as the BBB, is the most critical obstacle that must be overcome in order to produce new drugs for the CNS. Endothelial cells that are largely impermeable, have close connections, active efflux transport mechanisms, and enzymatic activity make up the BBB. It effectively prevents water-soluble molecules from entering the central nervous system, and it may also lower the concentration of lipid-soluble molecules in the brain by inhibiting the activity of enzymes or the activity of efflux pumps. Therefore, the BBB only allows a certain circulation of molecules necessary for neuronal activation.89
In food products
Amorphous silica NPs are used in pastes and powder products (like instant soups) to prevent caking. Amorphous silica is typically found in the form of the food additive E551. Additionally, it can be found in especially sunscreens in cosmetics. Sunscreens contain zinc oxide and titanium dioxide NPs because they are colorless and reflect and scatter Ultra Violet rays more effectively than bigger particles. Nanoparticles are undetectable due to their small size, which promotes consumer acceptance and, consequently, human skin protection against Ultra Violet-induced damage. As in the past, nanoparticles are still often used to enhance the appearance of materials. Automotive coatings are an excellent example because they have several layers. From laboratory discoveries to market products, the process frequently takes a very long time.23
In gene delivery
In this, Polynucleotide vaccines work by transferring relevant antigen-encoding genes to host cells. This causes the antigenic protein to be created close to expert antigen presentation cells and incites an immune response. Such vaccines provide both humoral and cell-mediated immunity because intracellular protein synthesis activates both arms of the immune system rather than extracellular protein deposition. Polynucleotide vaccines mostly consist of DNA, which is inexpensive to produce and has far better storage than the majority of protein-based vaccine components. NPs containing plasmid DNA may also be used as efficient sustained-release agents because of their fast transition from the degradative endo-lysosomal compartment to the cytoplasmic compartment. This technique for delivering genes could be exploited. To promote bone repair, PLGA NPs containing therapeutic genes, such as bone morphogen proteins, may be employed.90, 91
In oral delivery of peptides and proteins
In this, Advances in biotechnology and biochemistry have promoted the discovery of many bioactive substances and peptides and proteins vaccines. Since the bioavailability of these chemicals is constrained by gastrointestinal epithelial barriers and their vulnerability to gastrointestinal breakdown by digestive enzymes, the discovery of suitable carriers continues to be a challenge. Bioactive substances can be encapsulated and shielded from enzymatic and hydrolytic degradation with the help of polymeric nanoparticles. Protein or peptide distribution is impeded by a number of physiological and anatomical barriers in the gastrointestinal tract. For instance, the bacterial gut flora, pepsin, trypsin, and chymotrypsin in the gut lumen, proteolytic enzymes at the brush border membrane, the mucus layer, and the epithelial cell linlineself.83 The histological structure of the mucosa is intended to efficiently prevent in the gestion of environmental particulates. Delivering the medication in a colloidal carrier system is one important strategy for breaking the gastrointestinal barrier since it can increase the drug delivery system’s interaction mechanisms with the GI tract’s epithelial cells.92
Multidrug resistance reversal in cancer cells
Exosomes are extracellular vesicles (Evs) that are secreted by the majority of eukaryotic cells and play a role in intercellular communication. Exosomes transport proteins, DNA, mRNA, microRNA, long noncoding RNA, circular RNA, and other components that play important roles in regulating tumour growth, metastasis, and angiogenesis during the cancer development process, and can be used as a prognostic marker and/or grading basis for tumour patients, therapeutic targets, or even anticancer drug carriers. Their properties allow them to help important cancer hallmarks of cancer growth and spread. Cancer-derived exosomal trafficking has been detected in a variety of liquid and solid tumours, indicating their importance in cancer treatment.92 Due to cancer cells’ capacity to develop resistance mechanisms, anticancer drugs may only have a limited amount of effect against a range of solid tumor types, even when administered in the tumor interstitium.93 These mechanisms make cancers resistant to treatment. MDR, or multi-drug resistance, is the most significant problem in chemotherapy. MDR is largely brought on by an increase in plasma membrane glycoprotein (Pgp) expression, which has the ability to expel many positively charged xenobiotics from cells, including several anticancer medications. By bypassing Pgp-mediated MDR, several strategies, such as the use of colloidal carriers, have been employed to restore tumoral cells’ responsiveness to anticancer medications. It is thought that Pgp recognizes a drug to be refluxed out of tumoral cells only when it is present in the plasma membrane, rather than when it is located in the cytoplasm or lysosomes after endocytosis. This is the basis for drug association with colloidal carriers, such as NPs, to combat drug resistance. 95 They are found in all bodily fluids and are actively created in tumour cells, where they are secreted and it is used to promote tumour growth.
Table 7: Nanomaterials and nanoparticles clinically approved or proof-of-concept stages of research 96
|
“Nanoparticles |
Applications |
|
Metallic NPs: |
|
|
Iron oxide |
In Magnetic resonance imaging/cancer therapy |
|
Gold (nanorods, nanoshells, nanocages) |
In Cancer therapy, diagnosis |
|
Gold |
In vitro diagnostics/cancer therapy |
|
Carbon Structures NPs: |
|
|
Fullerenes |
In Cancer therapy |
|
Carbon nanotubes |
In Fluorescence and photoacoustic imaging, antioxidant |
|
Ceramic NPs: |
|
|
Silica |
In Cancer therapy, diagnosis |
|
Alumina |
In Cancer therapy, diagnosis, computed tomography |
|
Semiconductor Quantum dots |
In Fluorescent contrast, in vitro diagnostics |
|
Organic NPs: |
|
|
Protein based nanoparticles |
In Cancer therapy |
|
Polymer nanoparticles |
In Cancer therapy |
|
Polymer drug conjugates |
In Cancer therapy |
|
Polymeric micelles |
In Cancer therapy |
|
Dendrimers |
In Microbicides, cancer therapy |
|
Nanogels |
In Microbicides, cancer therapy |
|
Bicelles |
For Topical delivery |
|
DNA based nanoparticles |
In Cancer therapy |
|
Liposomes |
In Cancer therapy |
|
Hybrid NPs: |
|
|
Magnetoliposomes |
In Magnetic resonance imaging/cancer therapy” |
Marketed formulations of nanoparticles
some nanoformulations which are available in the market97
Table 8: Currently available nanoparticle formulations in the market.
|
“Product Name |
Company Name |
Drug \API |
ROA/ formulation |
Application |
Status in market |
|
Abraxane |
Abrasix bioscience, Astrazeneca |
Paclitaxel |
Albumin-bound nanoparticles/iv |
In Breast cancer |
Available in Marketed |
|
Caelyx |
Schering-Plough |
Doxorubicin |
Pegylated liposome/im |
In Breast and ovarian cancer, Kaposi sarcoma |
Available in Marketed |
|
Myocet |
Zeneus Pharma Ltd |
Doxorubicin |
Liposome/ iv |
In Breast cancer |
Available in Marketed |
|
Doxil |
Scquus Pharmaceuticals |
Doxorubicin |
Liposome/ iv |
In Kaposi sarcoma |
Available in Marketed |
|
Genexol-pm |
Samyang Pharmaceuticals |
Paclitaxel |
Methoxy PEG-PLA/iv |
In Breast and lung cancer |
Phase 2 |
|
CALAA-01 |
Calando Pharmaceuticals |
Anti-R2 SIRNA |
Cyclodextrin-containing polymer (CAL 101) and targeting agents (AD-PEG-TF)/iv |
In Solid tumors |
Phase 1 |
|
Rexin-G |
Epeius biotechnologies MD |
Dominant negative cyclin GI construct |
Pathotropic nanoparticle/iv |
Recurrent or metastatic breast cancer |
Phase ½” |
Conclusion
Nanotechnology is making our daily lives easier by increasing the performance of everyday objects through increased usefulness and efficiency. “It promotes environmental purity by providing better air and water as well as clean, renewable energy for a future that is more long-lasting than it would otherwise be. Leading universities, industries, and organizations are boosting their investment in research and development as a direct response to the growing interest in nanotechnology. As a result of the extensive research that is being done to bring nanotechnology into practice, this branch of science is currently at the leading edge. It is now being assessed for a variety of novel applications in the hopes of boosting the efficiency and performance of the product or process in question, which would, in turn, lead to a reduction in the price, making it more accessible to a wider audience. Because of its efficiency and low impact on the surrounding environment, nanotechnology has an exciting and bright future ahead of it.” Currently, nanoparticles are successfully employed to deliver medications. One of the most crucial instruments in nanomedicine, nanoparticles provide substantial advantages in medication targeting and delivery as well as the possibility for combination diagnostics and therapy. The following strategies are challenging to implement on a technical level: – Viral-like systems for intracellular systems, biomimetic polymer architecture, systems interacting with my body smart delivery, control of sensitive drugs, nanochips for NPs release, carriers for advanced polymers for peptide/protein delivery, and control of sensitive drugs are some examples of technologies that are currently being researched. Drug delivery systems were created in order to either distribute drugs or manage the amount and tempo at which they were administered. Formulations and dispersions including components with dimensions as tiny as nanometers are used in the majority of substantial and well-established internal drug delivery research initiatives. 98 Following a study of the previously conducted research, this work constructed a nanoparticle data storage. This page gives an overview of nanoparticles by classifying them, discussing the many methods for producing nanoparticles and discussing their many applications. According to the findings of our research, the prevalence of nanoparticles has quickly grown over the past several years. There are a large number of chances or possible projects that may be undertaken, and the costs of some of the manufactured nanoparticles are competitive. For instance, the synthesis of nanoparticles from plant resources has gained popularity in recent years due, among other things, to the fact that it is economical and kind to the environment.
Acknowledgement
IFTM University, Moradabad, Uttar Pradesh, India
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding sources.
References
- Pal D., Nayak A. K. Nanotechnology for targeted delivery in cancer therapeutics. Int J Pharm Sci Rev Res. 2010;1(1):1-7.
- Gupta S., Yadav B. S., Kesharwani R., Mishra K. P., Singh N. K. The role of nanodrugs for targeted drug delivery in cancer treatment. Arch Appl Sci Res. 2010;2(1):37-51.
- Hagens W. I., Oomen A. G., Jong W. H., Cassee, F. R., Sips, A. J. What do we (need to) know about the kinetic properties of nanoparticles in the body? Toxicol. Pharmacol. 2007;49(3):217-29.
- Couvreur P., Dubernet C., Puisieux F. Controlled drug delivery with nanoparticles: current possibilities and future trends. J. Pharm. Biopharm. 1995;41(1):2-13.
- Pal S. L., Jana U., Manna P. K., Mohanta G. P., Manavalan R. Nanoparticle: An overview of preparation and characterization. Appl. Pharm. Sci. 2011;228-234.
- Ando, The electronic properties of graphene and carbon nanotubes. NPG Asia Mater. 2009;17–21.
- Koçak A., Karasu B.General evaluations of nanoparticles. 2018;5(1):191-236.
- Nowack B., “Pollution Prevention and Treatment Using Nanotechnology”, In Krug, H.F., editor, Nanotechnology, Springer.
- Surender V., Deepika M. Solid lipid nanoparticles: a comprehensive review. J Chem Pharm Res. 2016; 8(8):102-14.
- Kumari, B. A Review on Nanoparticles: Their Preparation Method and applications. Ind Res J Pharm Sci.2018; 5(2):1420.
- Jahanshahi M., Babaei, Z. Protein nanoparticle: a unique system as drug delivery. Vehicles. 2016;7(25).
- Rawat M., Singh D., Saraf S. A. S. S., Saraf, S. Nanocarriers: promising vehicle for bioactive drugs. Pharm. Bull.2006;29(9):1790-1798.
- M. Research and Reviews: Int. J Pharm.
- Banach M., Szczygłowska R., Pulit J., Bryk M. Building materials with antifungal efficacy enriched with silver nanoparticles 2014.
- Konstantinović Z., Santiso J., Vodnik V., Saponjic Z., Nedeljkovic, J. Phyto-Synthesized Silver Nanoparticles: A Potent Biolarvicidal Agent. Nanomedicine Biotherapeutic Discov.2013; 3(112):2.
- Gupta D., Nguyen P., Yu M. Nanoparticles for superior pharmacokinetics and enhanced efficacy. J Dev Drugs.2014;3:2.
- Liu Z., Robinson J.T., Sun X., Dai H. PEGylated Nanographene Oxide for Delivery of WaterInsoluble Cancer Drugs. Am. Chem. Soc., 2008;130:10876-10877.
- Tiwari D. K., Behari J., Sen, P. Application of nanoparticles in wastewater treatment. World Appl Sci J.2008; 3(3):417-433.
- Sahoo B. M., Kumar,B. V. V. R., Patra Ch. N., Panda J. R., Mohanta B. C., Palei, N. N. https://doi.org/10.2174/24054615056662 Nanotechnology: A Novel Approach for Drug Development in the HealthCare System. Current Nanomaterials.2020;5(1):12-25.
- Meena J., Gupta A., Ahuja R., Singh M., Bhaskar S., Panda A. K. Inorganic nanoparticles for natural product delivery: A reviewChem. Lett.2008;18:2107-2118.
- Salavati-Niasari M., Davar F., Mir N. Synthesis and characterization of metallic copper nanoparticles via thermal decomposition. Polyhedron.2008;27(17):3514-3518.
- Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. J. Chem. 2019;12(7):908-931.
- Bhaviripudi S., Mile E., Steiner S. A., Zare A. T., Dresselhaus M. S., Belcher A. M., Kong, J. CVD synthesis of single-walled carbon nanotubes from gold nanoparticle catalysts. Am. Chem. Soc.2007;129(6):1516-1517.
- Varma M., Kumar K. T. S., Srivalli D. “A Review on nanoparticles: synthesis, characterization and applications” 2021;7(8),:169 – 179.
- Thakuria A., Kataria B., Gupta D. Nanoparticle-based methodologies for targeted drug delivery—an insight. J Nanopart Res.2021; 23(4).
- Tenne R. Fullerene-like materials and nanotubes from inorganic compounds with a layered (2-D) structure Colloids Surf. A: Physicochem. Eng. Asp.2002;208: 83–92.
- Huang X, Boey F., Zhang H U A. Graphene-nanoparticle composites Chem Soc Rev2010; 6:159–66.
- De Volder M. F., Tawfick S. H., Baughman R. H., Hart, A. J. Carbon nanotubes: present and future commercial applications. Science.2013;339(6119):535-539.
- Endo M., Kim Y. A., Hayashi T., Fukai Y., Oshida K., Terrones M., Dresselhaus M. Structural characterization of cup-stacked-type nanofibers with an entirely hollow core. Applied Physics Letters.2002; 80(7):1267-1269.
- Fawole O G., Cai X., Mackenzie A R. Gas flaring and resultant air pollution: A review focusing on black Environ. Pollut.2016;216:182–97
- Geetha P., Latha M S., Pillai S., Deepa B., Kumar K S., Koshy M. Green synthesis and characterization of alginate nanoparticles and its role as a biosorbent for Cr (VI) ions Mol. Struct. 2016; 1105:54–60
- Harshiny M., Iswarya C N., Matheswaran M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technol. 2015; 286:744–9
- Hulteen J C., Treichel D A., Smith M T., Duval M L., Jensen T R., Duyne R P Van. Nanosphere Lithography: Size-Tunable Silver Nanoparticle and Surface Cluster Arrays Phys. Chem. B 1999 ;103(19):3854–63
- Syed B., Prasad N M N., Satish S.Endogenic mediated synthesis of gold nanoparticles bearing bactericidal activity Microsc. Ultrastruct.2016;4:162–6
- Bau V. M., Bo X., Guo L. Nitrogen-doped cobalt nanoparticles/nitrogen-doped plate-like ordered mesoporous carbons composites as noble-metal free electrocatalysts for oxygen reduction reaction. Energy Chem.2017;26(1):63-71.
- Osuntokun J., Ajibade P. A. Morphology and thermal studies of zinc sulfide and cadmium sulfide nanoparticles in polyvinyl alcohol matrix. Physica B: Condensed Matter.2016;496: 106-112.
- Tyszczuk-Rotko K., Sadok I., Barczak M. Thiol-functionalized polysiloxanes modified by lead nanoparticles: synthesis, characterization and application for determination of trace concentrations of mercury (II). Microporous Mesoporous Mater.2016; 230:109-117.
- Ryu C., Joo S., Kim H. Two-step flash light sintering of copper nanoparticle ink to remove substrate warping Surf. Sci.2016; 384:182–91
- Bogutska К І., Sklyarov Y P., Prylutskyy Y І. Zinc and zinc nanoparticles: biological role and application in biomedicine 2013;1:9–16.
- Laad M., Jatti V K S.Titanium oxide nanoparticles as additives in engine oil KING SAUD Univ. – Eng. Sci.2016; 0–6.
- Ruales-lonfat C., Barona J F., Sienkiewicz A., Bensimon M., Vélez-colmenares J. Environmental Iron oxides semiconductors are efficients for solar water disinfection: A comparison with photo-Fenton processes at neutral pH Applied Catal. B, Environ. 2015;166-167:497–508.
- Kaynar Ü H., Çam S., Eral M. Modeling of thorium (IV) ions adsorption onto a novel adsorbent material silicon dioxide nano-balls using response surface methodology Appl Radiat Isot. 2016; 115:280–8.
- Bajpai S K., Jadaun M., Tiwari S. Synthesis, characterization and antimicrobial applications of zinc oxide nanoparticles loaded gum acacia / poly (SA) hydrogels Carbohydr Polym.2016;153:60–5.
- Kim S., Chung B H. Antioxidant activity of levan coated cerium oxide nanoparticles Polym.2016;150:400-407.
- Munuswamy D. B., Madhavan V. R., Mohan M. Synthesis and surface area determination of alumina nanoparticles by chemical combustion method. Int J ChemTech Res.2015;8: 413-419.
- Ealia S. A. M., Saravanakumar, M. P. A review on the classification, characterisation, synthesis of nanoparticles and their application. In IOP conference series: materials science and engineering 2017; 263:032019.
- Choudhury A., Laskar R. E., Deka D., Sonowal K., Saha S., Dey B. K. A review on nanoparticles: Types, preparation and its characterization. J. Pharm. Technol. 2021;14(3):1815-1822.
- Nikam A. P., Ratnaparkhiand M. P., Chaudhari, S. P. Nanoparticles–an overview. J. Res. Dev. Pharm. Life Sci.2014; 3:1121-1127.
- Boudad H., Legrand P., Lebas G., Cheron M., Duchene D., Ponchel, G. Combined hydroxypropyl-β-cyclodextrin and poly (alkylcyanoacrylate) nanoparticles intended for oral administration of saquinavir. J. Pharm.2001;218(1-2):113-124.
- Puglisi G., Fresta M., Giammona G., Ventura C. A. Influence of the preparation conditions on poly (ethylcyanoacrylate) nanocapsule formation. J. Pharm. 1995;125(2):283-287.
- Calvo P., Remunan‐Lopez C., Vila‐Jato J. L., Alonso M. J. Novel hydrophilic chitosan‐polyethylene oxide nanoparticles as protein carriers. Appl. Polym. Sci 1997;63(1):125-132.
- Kawaguchi S., Ito, K.Dispersion polymerization. Polymer Particles: -/-,2005; 299-328.
- Sugihara S., Blanazs A., Armes S. P., Ryan A. J., Lewis A. L. Aqueous dispersion polymerization: a new paradigm for in situ block copolymer self-assembly in concentrated solution. Am. Chem. Soc. 2007;133(39):15707-15713.
- Song C. X., Labhasetwar V., Murphy H., Qu X., Humphrey W. R., Shebuski R. J., Levy R. J. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. JCR.1997;43(2-3):197-212.
- Jaiswal J., Gupta S. K., Kreuter, J. Preparation of biodegradable cyclosporine nanoparticles by high-pressure emulsification-solvent evaporation process. JCR2004; 96(1):169-178.
- Soppimath K. S., Aminabhavi T. M., Kulkarni A. R., Rudzinski, W. E. Biodegradable polymeric nanoparticles as drug delivery devices. JCR. 2001;70(1-2):1-20.
- Tice T. R., Gilley R. M. Preparation of injectable controlled-release microcapsules by a solvent-evaporation process. JCR 1995; 2:343-352.
- Koosha F., Muller R. H., Davis S. S., Davies M. C.The surface chemical structure of poly (β-hydroxybutyrate) microparticles produced by solvent evaporation process.JCR1989; 9(2):149-157.
- Lemarchand C., Gref R., Passirani C., Garcion E., Petri B., Müller R., & Couvreur, P. Influence of polysaccharide coating on the interactions of nanoparticles with biological systems. 2006;27(1):108-118.
- Tabata Y., Ikada Y. Protein precoating of polylactide microspheres containing a lipophilic immunopotentiator for enhancement of macrophage phagocytosis and activation. Res.1989; 6:296-301.
- Allémann E., Gurny R., Doelker E. Drug-loaded nanoparticles: preparation methods and drug targeting issues. Eur J Pharm Biopharm.1993;39(5):173-191.
- Bodmeier R., Huagang C. Indomethacin polymeric nanosuspensions prepared by microfujidization. JCR.1990;12(3):223-233.
- Wang Y., Li P., Truong-Dinh Tran T., Zhang J., Kong, L. Manufacturing techniques and surface engineering of polymer-based nanoparticles for targeted drug delivery to cancer. Nanomater.2016;6(2):26.
- Vandervoort J., Ludwig A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design study. J. Pharm.2002;238(1-2):77-92.
- Ubrich N., Bouillot P., Pellerin C., Hoffman M., Maincent P. Preparation and characterization of propranolol hydrochloride nanoparticles: a comparative study. JCR.2004;97(2):291-300.
- Panigrahi D., Sahu P. K., Swain S., Verma R. K. Quality by design prospects of pharmaceuticals application of double emulsion method for PLGA loaded nanoparticles. SN Appl. Sci.2021; 3:1-21.
- Takeuchi H., Yamamoto H., Kawashima Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Drug Deliv. Rev. 2001;47(1):39-54.
- El-Shabouri M. H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. J. Pharm. 2002;249(1-2):101-108.
- Vargas A., Pegaz B., Debefve E., Konan-Kouakou Y., Lange N., Ballini J. P., Delie F. Improved photodynamic activity of porphyrin loaded into nanoparticles: an in vivo evaluation using chick embryos.J.Pharm.2004;286(1-2):131-145.
- Yoo H. S., Oh J. E., Lee K. H., Park, T. G. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Res. 1999; 16:1114-1118.
- Gazi A. S., Sailaja A. K. Preparation and Characterization of Paracetamol Loaded Eudragit S100 Nanoparticles by Salting Out Technique. Dev. Drugs. 2018;7(01):3-6.
- Tiruwa R. A review on nanoparticles-preparation and evaluation parameters. IJPBR 2016;4(2):27.
- Peltonen L., Koistinen P., Karjalainen M., Häkkinen A., Hirvonen J. The effect of cosolvents on the formulation of nanoparticles from low-molecular-weight poly (I) lactide. Aaps Pharmscitech.2002;3:52-58.
- Sharma D., Maheshwari D., Philip G., Rana R., Bhatia, S., Singh M., Dang S. Formulation and optimization of polymeric nanoparticles for intranasal delivery of lorazepam using Box-Behnken design: in vitro and in vivo evaluation. Biomed Res Int. 2014; 2014:156010
- Bohrey S., Chourasiya V., Pandey A. Polymeric nanoparticles containing diazepam: preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016;3(1):1-7.
- Laddha U. D., Kshirsagar S. J. Formulation of nanoparticles loaded in situ gel for treatment of dry eye disease: In vitro, ex vivo and in vivo evidences. Drug Deliv. Sci. Technol. 2021; 61:102112.
- Chishti N., Jagwani S., Dhamecha D., Jalalpure S., Dehghan M. H. Preparation, optimization, and in vivo evaluation of nanoparticle-based formulation for pulmonary delivery of anticancer drug. Med. 2019;55(6):294.
- Kesarla R., Tank T., Vora P. A., Shah T., Parmar S., Omri A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv. 2016;23(7):2363-2370.
- Betala S., Varma M. M., Abbulu, K. Formulation and evaluation of polymeric nanoparticles of an antihypetensive drug for gastroretention. JDDT2018;8(6):82-86.
- Aljaeid B. M., Hosny K. M. Miconazole-loaded solid lipid nanoparticles: formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int J Nanomedicine. 2016; 11:441.
- Kharia A. A., Singhai A. K., Verma R. Formulation and evaluation of polymeric nanoparticles of an antiviral drug for gastroretention. Int J Pharm Sci Nanotechnol, 2012;4(4):1557-1562.
- Couvreur P., Barratt G., Fattal E., Vauthier C. Nanocapsule technology: a review. Rev. Ther. Drug Carr. Syst.2002;19(2).
- Champeau Rachel. Assessing safety health risks of nanomaterials. 2006; 15:2005
- Jin Y., Huan Y., Zhao J. X., Wu M., Kannan S. Toxicity of nanomaterials to living cells. Proc Natl Acad Sci U S A.2005; 59:42-43.
- DelVecchio R. Berkeley considering need for nano safety. SF Chronicle.
- Shelake S. S., Patil S. V., Patil S. S. Formulation and evaluation of fenofibrate-loaded nanoparticles by precipitation method. Indian J. Pharm. Sci. 2018;80(3):420-427.
- Muthu M. S., Feng S.Pharmaceutical stability aspects of nanomedicines. Nanomed.2009;4(8):857-860. https://doi.org/10.2217/nnm.09.75
- Mohanraj V. J., Chen Y. J. T. J. O. P. R. Nanoparticles-a review. J. Pharm. Res. 2006;5(1):561-573.
- Chen Y., Dalwadi G., Benson H. A. E. Drug delivery across the blood-brain barrier. Drug Deliv.2004;1(4):361-376.
- Panyam J., Zhou W. Z., Prabha S., Sahoo S. K., Labhasetwar V. Rapid endo‐lysosomal escape of poly (DL‐lactide‐coglycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16(10):1217-1226.
- Tsai C. Y., Shiau A. L., Chen S. Y., Chen Y. H., Cheng P. C., Chang M. Y., Wu C. L. Amelioration of collagen‐induced arthritis in rats by nanogold. (AC&R) 2007;56(2):544-554.
- Jeon S. I., Lee J. H., Andrade J. D., De Gennes P. Protein—surface interactions in the presence of polyethylene oxide: I. Simplified theory. Colloid Interface Sci.1991;142(1):149-158.
- Krishna R., Mayer L. D. Multidrug resistance (MDR) in cancer: mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci2000;11(4):265-283.
- A. Löthman, Ayça Nur Demir, Cancer-derived Exosomes (CDE) and their Role in Nanomedicine, 5th Edition of Nanotechnology, Nanomedicine & Optics Photonics hybrid Conference, October 06-07 2022, Paris, France.
- Bennis S., Chapey C., Robert J., Couvreur, P. Enhanced cytotoxicity of doxorubicin encapsulated in polyisohexylcyanoacrylate nanospheres against multidrug-resistant tumour cells in culture. J. Cancer.1941;30(1):89-93.
- Horikoshi S., Serpone, N.Microwaves in nanoparticle synthesis: fundamentals and applications. John Wiley & Sons. 2013.
- Arunkumar. Development and Validation of New Analytical Methods for Simultaneous estimation of Epigallocatechin gallate, a component of Green Tea extractand Niacin in a Pharmaceutical dosage form, J. Pharm. Res., 2016; 5(2): 21-24.
- Lachman, Liberman Kaing. “Theory and practice of Industrial Pharmacy”, 3rd edn:26-30.

This work is licensed under a Creative Commons Attribution 4.0 International License.





