How to Cite | Publication History | PlumX Article Matrix
Sabeel Salam1* , Reshma Fathima4
, Reshma Fathima4 , P. P. Sreelekha3
, P. P. Sreelekha3 , M. Kavya5
, M. Kavya5  and E. Anjali2
and E. Anjali2
Department of pharmaceutics, Grace college of pharmacy, Palakkad, Kerala, India.
Corresponding Author E-mail: sabeelsalam1996@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3183
ABSTRACT: The ultimate objective about this experiment was to create a fast-dissolving tablet Isosorbide dinitrate that released its medication quickly. The physical properties of tablets, such as hardness, angle of repose, weight variation, and friability were assessed. In-vitro release was investigated, and a 22-factorial design was created. The results show that the in-vitro release shows that the F2 formulation has the highest release at 20 min. Tablet disintegration time ranges from 31 to 48 s. The hardness ranges from 6-7 kg/cm3. The friability test shows the range from 0.49-0.68%. The weight variation test shows that all the formulation passes the tests. ANOVA statistical analysis revealed that the generated formulations are statistically significant. It is concluded that FDT formulations have optimum and reproducible disintegration time and increased dissolve characteristics, resulting in higher patient compliance.
KEYWORDS: Angina Pectoris; Antianginals; Fast Dissolving Tablet; Isosorbide Dinitrate Therapy; Pharmacotherapy; Statistical Optimization
Download this article as:| Copy the following to cite this article: Salam S, Fathima R, Sreelekha P. P, Kavya M, Anjali E. Statistical Optimization of Fast Dissolving Tablet Contains Isosorbide Dinitrate for the Treatment of Angina Pectoris. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Salam S, Fathima R, Sreelekha P. P, Kavya M, Anjali E. Statistical Optimization of Fast Dissolving Tablet Contains Isosorbide Dinitrate for the Treatment of Angina Pectoris. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/49NWlgO |
Introduction
Angina pectoris, a cardiovascular ailment, is identified by chest pain or discomfort brought on by an alteration in the amount of blood flowing to the heart. One of the drugs used for the treatment of this condition is Isosorbide dinitrate (ISDN), which belongs to the class of nitrate drugs. In order to increase blood flow to the heart and lessen the strain on the heart, ISDN works through dilation of blood vessels 1.
Fast dissolving tablets (FDTs) have emerged as an attractive dosage form for the treatment of angina pectoris because they offer several advantages, such as rapid onset of action, improved patient compliance, and ease of administration 2.
The design and development of FDTs of ISDN involves various aspects such as the selection of suitable excipients, optimization of the formulation, and evaluation of the product for its physicochemical properties, in vitro drug release, and stability.
Several techniques such as direct compression, freeze-drying, and spray drying have been used for the preparation of FDTs of ISDN. The choice of technique depends on the physicochemical properties of ingredients utilised.
The development of FDTs of ISDN may involve the use of superdisintegrants which aid the tablet’s quick breakdown in the mouth. Other excipients such as mannitol, sorbitol, and lactose may be used as bulking agents or sweeteners 3,4.
Overall, the aim of the study was to develop a fast dissolving tablet contains Isosorbide dinitrate for the treatment of angina pectoris and designing and development of ISDN FDTs is an important field of research with the potential to improve angina pectoris treatment by providing a more convenient and effective dose form.
Materials and methods
Isosorbide dinitrate (Abbott, India), Microcrystalline cellulose (Yarrow chem, India), Sodium starch glycolate (Yarrow chem, India), Crocarmellose sodium (Yarrow chem, India), Talc (Yarrow chem, India).
Formula for a single tablet per batch required to create 250 mg of Isosorbide dinitrate tablets prepared using direct compression procedures shown (Table 1).
Table 1: Formulation of tablet by direct compression
|
Ingredients |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Isosorbide dinitrate |
10 |
10 |
10 |
10 |
10 |
10 |
|
Microcrystalline cellulose(MCC) |
100 |
90 |
80 |
100 |
90 |
80 |
|
Sodium starch glycolate(SSG) |
1% |
2% |
4% |
– |
– |
– |
|
Croscarmellose Sodium(CCS) |
– |
– |
– |
1% |
2% |
4% |
|
Mannitol |
100 |
110 |
120 |
100 |
110 |
120 |
|
Talc |
20 |
20 |
20 |
20 |
20 |
20 |
|
Magnesium stearate |
20 |
20 |
20 |
20 |
20 |
20 |
Precompression parameters
Angle of repose
The powder flow qualities were tested to identify whether the material flow was good or bad by performing this process in which powder was able to glide by means of a funnel to form a heap. A graph paper was placed beneath the funnel tip, and the radius and heights of the heap were measured. The angle of repose was computed by applying the formula 5:
tanθ = height/radius
Carr’s index
Flowability of powder was evaluated using a simple test that compared the poured density(bulk density) and the tapped density of powder 5.
carr’ s index = tapped densit – poured density X 100 tapped density
Hausner’s ratio
A proximate indicator of how easily powder flows is Hausner’s ratio. The following formula is used to compute it 5:
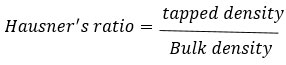
Bulk density and Tapped density
The bulk density is the ratio of the weight of the entire mass of the powder to the entire volume. It is supplied in g/ml and is represented as 5.
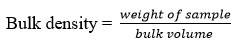
The blend’s weight was estimated using formula 5:
![]()
Post compression parameters
Weight variation
Twenty pills were chosen at random from each formulation, and The average mass was determined. Following that, the weights of several pills were compared to the mean weight 5.
Friability
Tablet friability was assessed using a friability test device. (Biolinkz, India). The weighted pills were placed in a friabilator (Weight initial). For 4 minutes, the friabilator was set to 25rpm. The tablets were dedusted and weighed once more. (Weight final). The percentage friability was calculated as follows 5:
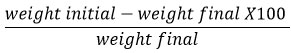
Hardness and Disintegration
Pfizer’s hardness tester has been utilized to gauge tablet’s-hardness, Whereas Disintegration test apparatus was used to assess the disintegration time of tablets 5.
In vitro dissolution study
The in vitro dissolution investigation was completed utilising a USP-type II device that revolves at 50 rpm. As the solution medium, pH buffered by phosphate (6.8) was used. The dissolving medium’s temperature was kept constant at 37.5°C. Shimadzu Double beam spectrophotometer at 277nm went to use to measure a quantity of the dissolution medium that was taken out at predetermined intervals, filtered, and measured 5.
Statistical Optimization
Optimization methods are used in the development process. They are typically used on tablets. The optimisation technique’s main goal is to determine the variable and quantify response with regard to variables in order to find the optimum. The normal protocols were used for optimization, and 22 with ∝ = 2 were used. The central point (0,0) was investigated in quintuplicate with the amount of Sodium starch glycolate (X1) and Mannitol (X2) as independent variables. The dependent variables were two responses: in vitro drug release (Y1) and disintegration time (Y2) 6.
Results and discussion
The results for pre compression parameters of Isosorbide dinitrate fast dissolving tablets of the formulation from F1 to F6, shows that the bulk density gives the ranges from 0.54-0.59 g/cm3. The Tapped density provides the range from 0.64-0.69 g/cm3. The Carr’s index provides the results of the ranges from 10.93-14.06%. The Hausner’s ratio provides the ranges from 1.12-1.19 and the results of angle of repose obtained from the ranges of 27.23-30.35. All the results exhibited on (Table 2).
Table 2: pre compression parameters
|
Formulation code |
Bulk-density |
Tapped-density |
Carr’s-index |
Hausner’s-ratio |
Angle of repose |
|
F1 |
059 |
0.68 |
13.23 |
1.15 |
30.13 |
|
F2 |
0.57 |
0.64 |
10.93 |
1.12 |
30.04 |
|
F3 |
0.58 |
0.66 |
12.12 |
1.13 |
27.16 |
|
F4 |
0.55 |
0.64 |
14.06 |
1.16 |
30.35 |
|
F5 |
0.54 |
0.65 |
13.96 |
1.17 |
28.35 |
|
F6 |
0.58 |
0.69 |
12.63 |
1.19 |
27.23 |
The results for post compression parameters of Isosorbide dinitrate fast dissolving tablets of the formulation from F1 to F6, shows that the hardness ranges from 6-7 kg/cm3. The friability test shows the range from 0.49-0.68%. The results of the weight variation test shows that all the formulation passes the tests. Disintegration profile of fast dissolving tablets of Isosorbide dinitrate shows the results from the ranges of 31-48 s, All the results exhibited on (Table 3). In vitro release of FDT of Isosorbide dinitrate shows the release ranges at 20 min was 84.40-98.85%, where all the results exhibited on (Table 4) and (fig. 1).
Table 3: post-compression parameters
|
Formulation code |
Hardness(kg/cm2) |
Friability(%) |
Weight variation |
Disintegration time(sec) |
|
F1 |
7.0 |
0.56 |
Pass |
45 |
|
F2 |
6.5 |
0.49 |
Pass |
31 |
|
F3 |
6.0 |
0.60 |
Pass |
40 |
|
F4 |
6.5 |
0.57 |
Pass |
39 |
|
F5 |
6.0 |
0.54 |
Pass |
42 |
|
F6 |
6.0 |
0.68 |
Pass |
48 |
Table 4: In-vitro releases
|
Time(min) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
55.30 |
79.21 |
60.66 |
57.12 |
61.07 |
75.12 |
|
5 |
63.04 |
86.44 |
70.90 |
65.74 |
71.40 |
80.21 |
|
10 |
71.02 |
91.05 |
76.66 |
74.41 |
73.60 |
86.45 |
|
15 |
78.11 |
94.80 |
84.02 |
81.30 |
80.90 |
91.85 |
|
20 |
84.40 |
98.85 |
91.74 |
88.50 |
89.74 |
96.44 |
 |
Figure 1: In vitro release of FDT Isosorbide dinitrate
|
Statistical Optimization which provides information after evaluation is that, 4 formulations corresponding to 22 factorial design of fast dissolving tablets. Values observed the 2 responses Y1 (in-vitro release), Y2 (Disintegration time). The selected independent variables X1 (Con:of sodium starch glycolate), X2 (Con:of Mannitol) were found to have 2 responses measured. All batches showed disintegration time (35-43), in vitro release (61.45-90.05).
Response(Y1)
in vitro release (Y1) = 71.07 – 2.518 sodium starch glycolate (X1) – 18.77 Mannitol (X2) – 4.182 sodium starch glycolate (X1) *Mannitol (X2)
Response(Y2)
disintegration (Y2) = 38.25- 3.250 sodium starch glycolate (X1) – 0.7500 Mannitol (X2) + 0.7500 sodium starch glycolate (X1) *Mannitol (X2)
The results of factorial design recommended only F2 formulation optimised combo of polymers that reached maximum desirability depending on dissolution studies of formulations and constraints applied. All the dates shown in (Table 3 to 6), surface plot shows if (fig.2 and 3) of both in vitro release and disintegration. Contour plot represents (fig. 4 and 5) where fig. 3: Contour plot of mannitol(X2) v/s sodium starch glycolate of disintegration release which shows that the dark shade in graph indicate the concentration of mannitol which is greater than 1 and fig. 2: Contour plot of mannitol(X2) v/s sodium starch glycolate of in-vitro release which shows that the dark shade in graph indicate the concentration of mannitol which is greater than 1, so through colour grade we can find the concentration of mannitol level.
Table 5: Regression Coefficients for Y2
|
Term |
Effect |
Coef |
SE Coef |
T-Value |
P-Value |
VIF |
|
Constant |
|
38.250 |
0.750 |
51.00 |
0.012 |
|
|
sodium starch glycolate (X1) |
-6.500 |
-3.250 |
0.750 |
-4.33 |
0.144 |
1.00 |
|
Mannitol (X2)
|
-1.500 |
-0.750 |
0.750 |
-1.00 |
0.500 |
1.00 |
|
X1*X2 |
1.500 |
0.7500 |
0.750 |
-6.00 |
0.750 |
1.00 |
Table 6: Analysis of disintegration in statistical optimization.
|
Source |
DF |
Adj SS |
Adj MS |
F-Value |
P-Value |
|
Model |
2 |
44.500 |
22.250 |
9.89 |
0.219 |
|
Linear |
2 |
44.500 |
22.250 |
9.89 |
0.219 |
|
sodium starch glycolate (X1) |
1 |
42.250 |
42.250 |
18.78 |
0.144 |
|
Mannitol (X2) |
1 |
2.250 |
2.250 |
1.00 |
0.500 |
|
X1*X2 |
1 |
2.250 |
2.250 |
-1.50 |
0.412 |
|
Error |
1 |
2.250 |
2.250 |
|
|
|
Total |
3 |
46.750 |
|
|
|
Table 7: Regression Coefficients for Y1
|
Term |
Effect |
Coef |
SE Coef |
T-Value |
P-Value |
VIF |
|
Constant |
|
71.07 |
4.18 |
16.99 |
0.037 |
|
|
sodium starch glycolate (X1) |
-5.04 |
-2.52 |
4.18 |
-0.60 |
0.655 |
1.00 |
|
Mannitol (X2) |
-37.54 |
-18.77 |
4.18 |
-4.49 |
0.140 |
1.00 |
|
X1*X2 |
-8.365 |
-4.182 |
4.18 |
-3.14 |
0.266 |
1.00 |
Table 8: Analysis of in-vitro in statistical optimization.
|
Source |
DF |
Adj SS |
Adj MS |
F-Value |
P-Value |
|
Model |
2 |
1434.23 |
717.11 |
10.25 |
0.216 |
|
Linear |
2 |
1434.23 |
717.11 |
10.25 |
0.216 |
|
sodium starch glycolate (X1) |
1 |
25.35 |
25.35 |
0.36 |
0.655 |
|
Mannitol (X2) |
1 |
1408.88 |
1408.88 |
20.13 |
0.140 |
|
X1*X2 |
1 |
69.97 |
69.97 |
20.13 |
0.143 |
|
Error |
1 |
69.97 |
69.97 |
|
|
|
Total |
3 |
1504.20 |
|
|
|
 |
Figure 2: Contour plot of mannitol(X2) v/s sodium starch glycolate, in-vitro.
|
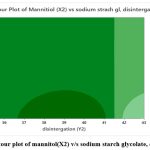 |
Figure 3: Contour plot of mannitol(X2) v/s sodium starch glycolate, disintegration.
|
 |
Figure 4: Surface plot of mannitol(X2) v/s sodium starch glycolate, disintegration
|
 |
Figure 5: surface plot of mannitol(X2) v/s sodium starch glycolate, in-vitro
|
Discussion
It is clear from a careful comparison with significant research and in-depth analysis that the formulations for quick-dissolving tablets were painstakingly created and rigorously tested with the main goal of achieving an expeditious release of isosorbide dinitrate. Examining carefully was done on significant tablet physical characteristics such bulk characterisation, angle of repose, weight fluctuation, hardness, and friability. Notably, weight homogeneity was obtained in all formulations, and the hardness and friability properties were substantially within the stated acceptable ranges. Additionally, the amount of the medication isosorbide dinitrate in every formulation was consistently within acceptable bounds, demonstrating a consistent dosage across all formulations. The F2 formulation in this study showed the most significant drug release after 20 minutes in a pH 6.8 phosphate buffer. The F2 formulation impressively showed an unparalleled drug release rate of 98.85% under these conditions. This unequivocally establishes the F2 formulation, enriched with 2% sodium starch glycolate, as the preeminent choice for the expeditious dissolution of isosorbide dinitrate tablets. The development of a fast-dissolving formulation of isosorbide dinitrate holds significant promise for patient-centered care. Individuals who may experience difficulty swallowing traditional tablets, such as elderly patients or those with certain medical conditions, could greatly benefit from this innovative formulation. This could lead to improved medication adherence and overall patient satisfaction. The convenience and ease of administration associated with fast-dissolving tablets have been shown to enhance patient adherence to prescribed medication regimens. For individuals with chronic conditions like heart disease, maintaining consistent treatment schedules is crucial for optimal outcomes. The F2 formulation could potentially address this aspect, leading to improved overall patient care.
Conclusion
In conclusion, the current investigation is an attempt to progress fast-acting tablets isosorbide dinitrate for the treatment of angina pectoris. By using different concentrations of Micro crystalline cellulose and Talc, six formulations for fast release of isosorbide dinitrate were created using the direct compression method. As superdisintegrants in F1 to F5, and F6 formulations, different amounts like Sodium starch glycolate(SSG) and Croscarmellose sodium(CCS) was chosen. ANOVA statistical analysis revealed that the generated formulations are statistically significant. F2 has the highest drug release (98.85%) at 20 min, hence it was chosen as the best formulation. The recommended fast dissolving formulations feature an optimal and reliable disintegration duration as well as improved dissolve characteristics, resulting in improved patient compliance.
Acknowledgment
I would like to express my deepest gratitude towards Gokulnath Kathiresan, friends and family for their support.
Conflict of Interest
The authors have reported no conflicts of interest regarding this investigation.
Funding Source
No funding source accepted for this investigation.
References
- Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, Bhat G. A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. New England Journal of Medicine. 1991 Aug 1;325(5):303-10.
CrossRef - Panigrahi R, Behera S. A Review on Fast Dissolving Tablets. Webmedcentral Quality and Patient Safety (2010) 1(9), 1-15.
- Debjit Bhowmik, Chiranjib, Jyoti jaiswal, Vinod Dubey, Margret Chandira. Fast dissolving tablet: A review on revolution of novel drug delivery systems and new market opportunities. Scholars Research Library- Der Pharmacia Lettre, 2009, 1(2), 262-276.
- Basawaraj.S.Patil, Mahesh.M.Gada, Upendra Kulkarni, K.Dayakar Rao. Formulation and Development of Candesartan Cilexetil Fast Dissolving Tablets by Solid dispersion technique. International Journal of Drug Formulation and Research (2011) 2(3), 233-247.
- Aulton ME, Taylor K, editors. Aulton’s pharmaceutics: the design and manufacture of medicines. Elsevier Health Sciences; 2013, 336.
- Kaffash E, Saremnejad F, Abbaspour M, Mohajeri SA, Garekani HA, Jafarian AH, Sardo HS, Akhgari A, Nokhodchi A. Statistical optimization of alginate-based oral dosage form of 5-aminosalicylic acid aimed to colonic delivery: In vitro and in vivo evaluation. Journal of Drug Delivery Science and Technology. 2019 Aug 1;52:177-88.
CrossRef - Md. Semimul Akhtar. Formulation and characterization of fast dissolving tablets along with antiepileptic drugs. World Journal of Pharmaceutical Research (2020) 9(4), 547-561.
- Rajni Bala, Sushil Khanna, Pravin Pawar. Polymers in Fast Dissolving Tablets-A Review. Asian Journal of Pharmaceutical and Clinical Research. (2012) 5(2), 8-14.
- Md Tarique Iqbal, Ishaat Hussain, Rajeshree Panigrahi. Enhancement of Solubility for Risperidone Fast Dissolving Tablets. International Journal of Universal Pharmacy and Life Sciences. (2012) 2(3), 394-408.
- N.L.Prasanthi, S.S.Manikiran, N.Rama Rao. Formulation and Characterization of Fast Dissolving Tablets of Raloxifene Hydrochloride. International Journal of Pharmaceutical Sciences and Drug Research. (2010) 2(1), 55-57.
- P.Ashish, M.S.Harsoliya, J.K.Pathan, S.Shruti. A Review- Formulation of Mouth Dissolving Tablets. International Journal of Pharmaceutical and Clinical Science. (2011) 1(1), 1-8.
- Prashant B Pawar, Avinash G Mansuk, Kuldip H Ramteke, Y P Sharma, Sagar N Patil. Mouth Dissolving Tablet: A Review. International Journal of Herbal Drug Research, (2011) 1(2), 22-29.
- Dali Shukla, Subhashis Chakraborty,Sanjay Singh, Brahmeshwar Mishra. Mouth Dissolving Tablets I: An Overview of Formulation Technology. Sci Pharm (2009) 76, 309-326.
CrossRef - Sofia A. Papadimitriou, Panagiotis Barmpalexis, Evangelos Karavas, Dimitrios N. Bikiaris. Optimizing the ability of PVP/PEG mixtures to be used as appropriate carriers for the preparation of drug solid dispersions by melt mixing technique using artificial neural network, European Journal of Pharmaceutics and Biopharmaceutics. (2012) 1-12.
CrossRef - Venkata Ramana Reddy, Sathyanarayana Dondeti, Manavalan.R, Sreekanth.J. Comparison of lyophilisation and compression technique of Risperidone oral disintegrating tablets. Der Pharma Chemica. (2010) 2(2), 172-184.
- 16. Ved Prakash, Saurabh Maan, Deepika, Shiv Kumar Yadav, Hemlata, Vikas Joogpal. Fast disintegrating tablets: Opportunity in drug delivery system. Journal of Advanced Pharmaceutical Technology & Research. (2011) 2(4), 223-235.
CrossRef - Jaysukh J Hirani, Dhaval A Rathod, Kantilal R Vadalia. Orally Disintegrating Tablets: A Review. Tropical Journal of Pharmaceutical Research, (2009) 8(2), 161-172.
CrossRef - Lokesh Puttalingaiah,Kunchu Kavitha,Tamizh Mani.T. Fast disintegrating tablets: An overview of Formulation, technology and evaluation. Research Journal of Pharmaceutical, Biological and Chemical Sciences. (2011) 2(2), 589-601.
- Zhang F, Zhou Y, Wu N, Jia R, Liu A, Liu B, Zhou Z, Hu H, Han Z, Ye X, Ding Y. In silico prediction of bioequivalence of Isosorbide Mononitrate tablets with different dissolution profiles using PBPK modeling and simulation. European Journal of Pharmaceutical Sciences. 2021 Feb 1;157:105618.
CrossRef - Alami-Milani M, Salatin S, Nasiri E, Jelvehgari M. Preparation and optimization of fast disintegrating tablets of isosorbide dinitrate using lyophilization method for oral drug delivery. Therapeutic Delivery. 2021 May;12(7):523-38.
CrossRef - Ratnaparkhi MP, Mohanta GP, Upadhyay L. Review on: Fast dissolving tablet. Journal of pharmacy research. 2009 Jan;2(1):5-12.
- Muzawar HM, Chandur VK, Shabaraya AR. Formulation evaluation and optimization of fast dissolving oral strips of isosorbide mononitrate. AJPTR. 2014;4(3):454-71.
- Li ZQ, He X, Gao X, Xu YY, Wang YF, Gu H, Ji RF, Sun SJ. Study on dissolution and absorption of four dosage forms of isosorbide mononitrate: Level A in vitro–in vivo correlation. European journal of pharmaceutics and biopharmaceutics. 2011 Oct 1;79(2):364-71.
CrossRef - Shotan A, Brill Z, Matetzky S, Shachar A, Feigenberger Z, Hod H, Kaplinsky E. Pain Scoring–A Method for Assessing Acute Antianginal Therapy Comparison of the Response to Acute Sublingual Administration of an Isosorbide Dinitrate Tablet, Isosorbide Dinitrate Spray and Nitroglycerin Spray in Unstable Angina. Cardiology. 1998 Mar 16;89(3):163-9.
CrossRef - Reisin LH, Landau E, Darawshi A. More rapid relief of pain with isosorbide dinitrate oral spray than with sublingual tablets in elderly patients with angina pectoris. The American Journal of Cardiology. 1988 Mar 25;61(9):2-3.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





