How to Cite | Publication History | PlumX Article Matrix
Antagonistic Behavior of Streptomyces chartreuse against Pathogenic Bacteria in Ricinus communis L.
Bhoomi N. Patel* , Priti Patel
, Priti Patel and Gayatri Patel
and Gayatri Patel
Department of Microbiology, Mehsana Urban Institute of Sciences, Ganpat University, Kherva, Gujarat.
Corresponding Author E-mail: bhoomipatel2917@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3214
ABSTRACT: Antibiotics are a crucial tool in modern medicine and have saved countless lives by effectively treating a wide range of bacterial infections. The microbial antibiotic have several biotechnological applications viz. agriculture, pharmaceuticals, food preservation, animal nutritions. The diverse array of applications and the various roles of bioactive metabolites produced by Actinomycetes have sparked a growing interest in the exploration of unique and unprecedented Actinomycetes strains. The Actinomycetes from soil ecosystem, marine ecosystem, rhizosphere of plant roots are also known to secrete novel antibiotics. In this context, the main objective of this research is to isolate and screen Actinomycetes strains that are capable of producing highly potent culturable secondary metabolites with novel antibacterial properties. These metabolites can potentially serve as biocontrol agents against Xanthomonas infections in Ricinus communis L., offering uncommon and innovative applications within the field of agriculture. All the Actinomycetes isolates were isolated from Mehsana regions of Gujarat an area of over 4,401 km2, with wide microbial diversity and can serve as a source for promising antibiotics producers. 7 rhizospheric soil samples were collected from various region sites viz. Ranasan, Mansa, Panchot, Gozariya, Kansa, Langhnaj, and Kherva. Total 76 antibiotic producing Actinomycetes isolates were obtained in Primary Screening. Based on the results of primary screening, potential morphologically diverse 3 isolates were selected for antibiotic production in liquid medium. FTIR analysis of three samples revealed distinct bands in the spectra. Sample-1 exhibited O-H (1347 cm^-1) and C-N (1191 cm^-1) groups. Sample-2 displayed O-H (3462 cm^-1), C-O (1043 cm^-1), and C=O (1736 cm^-1) groups. Sample-3 showcased O-H (3466 cm^-1), C=O (1737 cm^-1), C-N (1232 cm^-1), and C-O (1043 cm^-1) groups, providing valuable insights into their chemical compositions. The isolate BNPA72 gave best antibiotic production and was identified as Streptomyces chartreusis by 16 s rRNA gene sequencing method. The isolate Streptomyces chartreusis BNPA72 was able to inhibit the plant pathogen Xanthomonas, hence categorized as Biocontrol agents.
KEYWORDS: Actinomycetes; primary screening; secondary screening; Streptomyces sp
Download this article as:| Copy the following to cite this article: Patel B. N, Patel P, Patel G. Antagonistic Behavior of Streptomyces chartreuse against Pathogenic Bacteria in Ricinus communis L. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Patel B. N, Patel P, Patel G. Antagonistic Behavior of Streptomyces chartreuse against Pathogenic Bacteria in Ricinus communis L. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/47FoR1K |
Introduction
The group of Gram-positive prokaryotic bacteria known as Actinomycetealas can be distinguished from other groups because their DNA has a high concentration of guanines and cytosines. Actinomycetes exhibit a diverse array of morphologies, with some of the more extensively studied members, such as the odour-producing species found in the Streptomyces genus, displaying a filamentous vegetative form that may superficially resemble fungal hyphae. It is important to note that Actinomycetes are not fungi but rather a distinct group of microorganisms. The order Actinomycetales, to which filamentous Streptomycetes belong, encompasses a wide variety of morphological types, including single-celled rod-shaped cocci, hyphae that fragment, and branched mycelia.
This classification lumps together a collection of organisms with dissimilar morphologies and ecological roles under a single order. Many of the bacteria classified within Actinomycetales have no direct association with the production of water odors. In reality, these bacteria are isolated from a broad range of terrestrial and aquatic ecosystems. However, due to their slow growth and notorious difficulty in laboratory cultivation, our understanding of their distribution, ecology, and their capacity to thrive and adapt to diverse environments remains largely incomplete. This information is essential for our ability to identify the sources of taste and odor issues in water and differentiate between potential terrestrial and aquatic origins. 1. The remarkable adaptability of Actinobacteria to thrive in a wide range of ecological settings establishes them as prevalent filamentous microorganisms. They are capable of thriving in extreme environmental conditions and are classified into categories such as psychrophilic, thermophilic, alkaliphilic, acidophilic, and halophilic. Actinobacteria represent a valuable reservoir of diverse compounds, encompassing antibiotics, secondary metabolites, and enzymes of great industrial significance.
The inherent capacity of Actinobacteria to decompose a wide array of substrates, convert organic compounds, and transform agricultural and urban waste materials into products with added value holds substantial economic potential. The utilization of Actinomycetes spans a variety of fields, including medicine, biotechnology, industry, and environmental applications, and this has been extensively documented, marking it as a thriving avenue of research. Actinobacteria members emerge as a promising source of a diverse spectrum of enzymes essential for the degradation of lignocellulose, lignin, cellulose, and plant residues. 2 . A novel microorganism was identified using an isolation technique that involved (i) Selecting microorganisms capable of thriving on a soil extract agar medium instead of a conventional growth medium and (ii) spotting microscopic microbial colonies using a microscope. For isolation of Actinomycetes pre-treatment is mandatory to remove unrequired microorganisms from the medium. In some instances, phenol treatment is also a method to get rid of contaminant microorganisms. But here when phenol is applied, the growth of some phenol sensitive bacteria is stifled which might be crucial to your study. Hence, for best results pre-treatment is the best option. 3.
The “golden era” of antibiotic discovery, spanning from 1950 to 1970, witnessed the successful commercialization of numerous life-saving antibiotics, including streptomycin, vancomycin, rifamycin, and others. However, in the subsequent decades, the re-discovery of already known compounds and the technical challenges associated with purifying and understanding the structures of new compounds led to a significant decline in traditional research efforts. Despite the apparent decrease in research on microbial natural products, several academic research groups are persistently engaged in advancing the field. They are actively exploring new techniques for sampling and isolating potential Actinomycetes from previously uncharted sources. This ongoing effort serves to reduce the risk of rediscovering known compounds and enhances the availability of diverse Actinomycetes. These initiatives are crucial for the sustained advancement of Actinomycetes research over the long term. 4-5.
Isolation of Actinomycetes
Soil samples were collected from various seven sites, including Ranasan (23.5296 ͦ N, 72.7540 ͦ E), Mansa (23.4350 ͦ N, 72.6565 ͦ E), Panchot (23.6267 ͦ N, 72.3356 ͦ E), Gozariya (23.4792 ͦ N, 72.5648 ͦ E), Kansa (23.7081 ͦ N, 72.5160 ͦ E), Langhnaj (23.4471 ͦ N, 72.4983 ͦ E), and Kherva (23.5442 ͦ N, 72.4421 ͦ E) in the Mehsana District of Gujarat India. These dwellings contained a sample of rhizospheric soil [6]. At a depth of 5 cm, rhizospheric soil of Ricinus plants was sampled. 7. Serial dilution and Streak plate techniques were used to isolate Actinomycetes. Soil sample were serially diluted to 10 -1 to 10 -6 with sterile distilled water and subsequently 0.1ml of each dilutions was spread on SCA (Starch Casein broth). The plates were incubated at 32°C for 6-7 days. The modified SCA medium consists soluble starch – 10 g, Ferrous sulfate – 0.01 g, inoculated Magnesium sulfate – 0.05 g, Potassium nitrate – 2 and supported maximum numbers of Actinomycetes sp. Hence was used for further analysis. 8-9-10-11 .
Isolation of plant pathogens
Leaves from Ricinus plants showing signs of bacterial black spot were typically infected with Xanthomonas sp. To investigate the infection, the infected leaves were crushed using a sterilized mortar and pestle. The crushed leaves were then placed into Nutrient broth and kept at a temperature of 37°C for 24-48 hours under sterile conditions. After the incubation period, the mixture was transferred onto Nutrient agar plates and further incubated at 37°C for 24-48 hours. This process allowed for the growth and analysis of bacterial colonies. 14-15. For additional research, Bacterial culture was transferred into a sterile Nutrient agar slant and kept there in refrigeration conditions for subsequent study. 16.
Primary screening of Actinomycetes against plant pathogens
Using Mueller-Hinton agar, the cross-streak method was used to determine the antibacterial activity of pure isolates. 20-21. Actinomycetes cultures were prepared, and Mueller-Hinton agar was then streaked with the inoculum to protect it from pathogenic bacteria. All plates were incubated at 37°C for 24-48 hours after bacteria inoculation. 12-22-23. Antagonism was observed by the inhibition by plant pathogen in Muller-Hinton agar. 24.
Secondary screening of isolates
Potent efficacy of Streptomyces chartreusis against plant pathogens was shown by some isolates in primary screening. 25. Using the Agar well diffusion method, further Actinomycetes antibacterial activity was evaluated. 26. The potential Actinomycetes isolates based on primary screening results were grown in Erlenmeyer flasks containing starch casein broth, incubate flasks in shaking condition at 32˚C at 150rpm for 6 to 7 days. Further broths were centrifuged at 10,000 rpm for 30mins. 27. The supernatants were used to study antibacterial activities of potent isolates against Plant pathogen Xanthomonas sp.
Morphological characteristics of selected Actinomycetes and plant pathogens
Morphological characteristics were examined under a microscope, considering factor such as size, shape, arrangement and gram reaction. Additionally cultural characteristics were evaluated based on parameters like size, shape, arrangement, border, elevation, texture, moisture and pigment. 17-18-19.
Molecular identification of selected Actinomycetes and plant pathogens
Out of 76 potent antibiotic producers, high antibiotic producing Actinomycetes isolate BNPA72 was selected for further study. Isolation and quality of genomic DNA was chacked on 1.0% Agarose Gel. Subsequently, PCR was employed to amplify a segment of the 16S rRNA gene, which was then identified through sanger sequencing. 28-29.
FTIR analysis of crude extracts from Actinomycetes
The analysis of functional groups in the FP (presumably a sample or substance) was conducted using Fourier Transform –Infrared spectroscopy (FTIR) with a FTIR-Bruker alpha model. This analysis involved a powder form or aqueous form, where spectra of the sample in aqueous form were recorded using the FT-IR spectrophotometer. These spectra were collected within the range of 400 to 4000 cm^-1 with a spectral resolution of 4 cm^-1. 29-30.
Result and discussion
Screening of Actinomycetes for antibacterial activity against plant pathogen Xanthomonas in Ricinus communis L.
Table 1: Primary screening by using cross streak method
|
Sr.no |
Name of Isolates |
Activity against plant pathogens |
|
1 |
BNPA2 |
+ |
|
2 |
BNPA4 |
+ |
|
3 |
BNPA6 |
+ |
|
4 |
BNPA8 |
+ |
|
5 |
BNPA9 |
+ |
|
6 |
BNPA10 |
+ |
|
7 |
BNPA13 |
+ |
|
8 |
BNPA36 |
+ |
|
9 |
BNPA41 |
+ |
|
10 |
BNPA44 |
+ |
|
11 |
BNPA45 |
+ |
|
12 |
BNPA46 |
+ |
|
13 |
BNPA47 |
+ |
|
14 |
BNPA50 |
+ |
|
15 |
BNPA51 |
+ |
|
16 |
BNPA55 |
+ |
|
17 |
BNPA57 |
+ |
|
18 |
BNPA61 |
+ |
|
19 |
BNPA62 |
+ |
|
20 |
BNPA65 |
+ |
|
21 |
BNPA68 |
+ |
|
22 |
BNPA72 |
+ |
Secondary screening of Actinomycetes for antibacterial activity against plant pathogen Xanthomonas in Ricinus communis L.
 |
Figure 1: Agar well diffusion method against plant pathogen Xanthomonas.
|
Table 2: Zone measurement in agar well diffusion method
|
Sr.no |
Name of Isolates |
Zone of inhibition (mm ± S.D) |
|
1 |
BNPA61 |
39.82 ± 0.05 |
|
2 |
BNPA67 |
37.05 ± 0.06 |
|
3 |
BNPA72 |
29.05 ± 0.06 |
|
4 |
Kanamycin |
22.22 ± 0.05 |
|
5 |
Tetracycline |
26.02 ± 0.05 |
Table 3: Morphological characteristic of potent antibiotic producing Actinomycetes on SCA medium
|
Morphological characteristic of selected Actinomycetes colony |
||||
|
Isolates |
Aerial mycelium |
Substrate mycelium |
Diffusible pigment |
Gram- staining |
|
BNPA61 |
Light grey |
cream |
yellow |
Gram positive |
|
BNPA67 |
Dark grey |
Black |
Light brown |
Gram positive |
|
BNPA72 |
Grey |
White |
No diffusion |
Gram positive |
Table 4: Morphological characteristic of plant pathogen Xanthomonas on Nutrient agar medium
|
Morphological characteristic of Xanthomonas colony |
||||
|
Isolates |
Pigment |
Appeareance of Colony surface |
Mucoidness |
Plant pathogens |
|
BNPP11 |
Light grey |
cream |
yellow |
Gram positive |
Table 5: Cultural characteristic of selected Actinomycetes colony
|
Cultural characteristic of selected Actinomycetes colony |
||||
|
Isolates |
Aerial mycelium |
Substrate mycelium |
Diffusible pigment |
Gram- staining |
|
BNPA61 |
Light grey |
cream |
yellow |
Gram positive |
|
BNPA67 |
Dark grey |
Black |
Light brown |
Gram positive |
|
BNPA72 |
Grey |
White |
No diffusion |
Gram positive |
 |
Figure 2: FTIR analysis of BNPA72 Streptomyces chartreusis
|
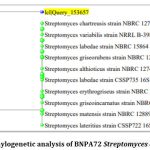 |
Figure 3: Phylogenetic analysis of BNPA72 Streptomyces chartreusis
|
Discussion
Present study focused on isolation of secondary metabolites producers bacteria from rhizospheric soil of Ricinus communis L. Isolation of Actinomycetes was done by using four sector method, purified colony classified based on morphological characteristics like aerial mycelia and substrate mycelia in different medium like Starch casein agar, Actinomycetes isolation agar, and Starch M-Protein Agar slant. Isolation of plants pathogen was done by four sector method and purified colony classified based on morphological characteristics like yellow pigment, Mucoidness.
Primary screening was used to categorise antibacterial Actinomycetes using the cross streak method. In cross streak method in the center of plate one line of pathogenic bacteria Xanthomonas sp. and its opposite direction streak Actinomycetes and observed Minimum inhibitory concentration in plates. There were total 76 Actinomycetes, from them 22 gave positive result in primary screening against plant pathogens.
The secondary screening of Actinomycetes against plant disease in Ricinus communis L. are determined by using Cross streak method and Agar well diffusion method. The diameter of the clear zone observed 39.82mm, 37.05mm and 29.05mm in BNPA61, BNPA67, BNPA72 by using Agar well diffusion method (Table-2).
Figure-2 this study conducted Fourier Transform Infrared Spectroscopy (FTIR) analysis on three distinct samples (Sample-1, Sample-2, Sample-3) within the spectral range of 400 to 4000 cm^-1 to identify characteristic functional groups. The FTIR spectra revealed specific bands indicative of functional groups in each sample. Sample-1 exhibited distinguishing bands at 1347 cm^-1, indicating the presence of (O-H groups) and at 1191 cm^-1 (C-N groups). Sample-2 exhibited distinguishing bands at 3462 cm^-1, (O-H groups) and at 1043 cm^-1 (C-O groups) and 1736 cm^-1 (C=O groups). Sample-3 featured bands at 3466 cm^-1, (O-H groups), 1737 cm^-1 (C=O groups), 1232 cm^-1 (C-N groups). The FTIR analysis of Streptomyces chartreuse highlights significant functional groups, including carbonyl, hydroxyl, and amino groups. These findings suggest the presence of key structural components in the antibiotics produced. Carbonyl groups are indicative of potential antimicrobial ketones or aldehydes, while hydroxyl groups may signify the involvement of bioactive alcohols or phenols. The identified amino groups hint at the presence of essential building blocks like amino acids or peptides, collectively contributing to the antagonistic behavior by inhibiting the growth of competing microorganisms, thereby elucidating the molecular basis of antibiotic production.
The results of the 16S rRNA gene sequencing (Figure-3) indicate that your isolate BNPA72 is highly similar to several known strains of Streptomyces, specifically Streptomyces chartreusis strain NBRC 12753, Streptomyces variabilis strain NRRL B-3984, Streptomyces labedae strain NBRC 15864, Streptomyces erythrogriseus strain NBRC 14601, Streptomyces matensis strain NBRC 12889, Streptomyces griseoincarnatus strain NBRC 12871, Streptomyces griseorubens strain NBRC 12780, Streptomyces althioticus strain NBRC 12740, Streptomyces labedae strain CSSP735, and Streptomyces lateritius strain CSSP722. The similarity between your isolate and these known strains is approximately 99.51%.
Actinorhodin production by Streptomyces coelicolor is extensively documented, with reported concentrations ranging from tens to hundreds of milligrams per liter (mg/L) under laboratory conditions. Similarly, erythromycin production by Streptomyces erythreus can vary, typically falling within the range of 100 to 1000 mg/L in optimized fermentation settings. Notably, these figures are general approximations, subject to variations contingent upon specific strains, fermentation parameters, and the particular antibiotic in question. For Streptomyces sp. SM01, isolated from Indian soil and noted for producing a novel antimicrobial compound, the observed concentration was found to be 0.01 µg/ml. It is imperative to recognize that these quantities are indicative, and actual yields may fluctuate depending on distinct microbial strains, cultivation conditions, and the specific nature of the synthesized antibiotic.
The study may not fully capture the impact of varying environmental conditions on antibiotic production. Future research incorporating diverse environmental parameters could enhance the ecological relevance of the findings.
The antagonistic behavior of Streptomyces chartreuse, as revealed in this study, holds significant ecological implications, particularly in the rhizospheric ecosystem. Its ability to produce antibiotics suggests a potential role in shaping microbial communities and suppressing pathogenic organisms in the soil. In agriculture, harnessing the antagonistic properties of Streptomyces chartreuse could contribute to sustainable pest and disease management practices, promoting healthier plant growth. Furthermore, the findings open avenues for biotechnological applications, wherein the strain’s bioactive compounds may find use in developing novel antimicrobial agents or biocontrol strategies for enhanced crop protection.
Conclusion
Actinomycetes, which are known for producing antibiotics, are widely found in soil environments. These Actinomycetes generate new antibiotics that serve various roles in the environment, exhibit bioactive properties, and have extensive applications in biotechnology. However, the microbial diversity in the Mehsana region of Gujarat remains mostly untapped concerning antibiotic-producing organisms and their potential applications. This study sheds light on the presence of diverse and promising antibiotic producers in the rhizospheric soil of Mehsana, Gujarat. We isolated 22 antibiotic producers from 7 different sites, with the maximum antibiotic producers being obtained from Ranasan. After secondary screening, 3 antibiotic producers with significant potential were identified, with isolate BNPA72 standing out as the most proficient antibiotic producer. This particular isolate was determined to be Streptomyces chartreusis BNPA72. Streptomyces antibiotics have been utilized as biocontrol agents, promote plant growth, and produce certain organic compounds used as fertilizer to improve crop productivity. Moreover, the antibiotics produced by Streptomyces can effectively inhibit the growth of plant pathogens like Xanthomonas, leading to enhanced crop productivity in Ricinus communis L. Consequently, the antibiotic produced by Streptomyces chartreusis BNPA72 holds promise for a wide range of applications. Notably, its antibiotic yield surpasses the currently reported yields. Furthermore, it is possible to enhance the production of antibiotic by Streptomyces chartreusis BNPA72 through media optimization and recombinant DNA technology.
Acknowledgment
We are very thankful to the management of Ganpat University and MUIS for providing their kind support and also for the availability of needed resources.
Conflict of Interest
There are no conflicts of interest.
Funding Sources
There is no funding sources.
References
- Zaitlin and S. B. Watson, “Actinomycetes in relation to taste and odour in drinking water: Myths, tenets and truths,” Water Res., vol. 40, no. 9, pp. 1741–1753, 2006, doi: 10.1016/j.watres.2006.02.024.
- Salwan and V. Sharma, The Role of Actinobacteria in the Production of Industrial Enzymes. Elsevier B.V., 2018. doi: 10.1016/B978-0-444-63994-3.00011-4.
- Hamaki et al., “Isolation of novel bacteria and actinomycetes using soil-extract agar medium,” J. Biosci. Bioeng., vol. 99, no. 5, pp. 485–492, 2005, doi: 10.1263/jbb.99.485.
- A. Jose and B. Jha, “New dimensions of research on actinomycetes: Quest for next generation antibiotics,” Front. Microbiol., vol. 7, no. AUG, pp. 1–5, 2016, doi: 10.3389/fmicb.2016.01295.
- Risandiansyah and A. Yahya, “Antibiotic activity of actinomycetes isolated from young tectona grandis (L.) wood and pith,” Biointerface Res. Appl. Chem., vol. 12, no. 6, pp. 8174–8183, 2022, doi: 10.33263/BRIAC126.81748183.
- Singh et al., “Isolation, screening, and identification of novel isolates of actinomycetes from India for antimicrobial applications,” Front. Microbiol., vol. 7, no. DEC, 2016, doi: 10.3389/fmicb.2016.01921.
- Ganesan et al., “Antimicrobial activity of some actinomycetes from Western Ghats of Tamil Nadu, India,” Alexandria J. Med., vol. 53, no. 2, pp. 101–110, 2017, doi: 10.1016/j.ajme.2016.03.004.
- Abdelrahman et al., “Evaluating the Antagonistic Potential of Actinomycete Strains Isolated From Sudan’s Soils Against Phytophthora infestans,” Front. Microbiol., vol. 13, no. June, 2022, doi: 10.3389/fmicb.2022.827824.
- A. Hassan and A. F. Mahmoud, “Isolation, Phenotypic and Molecular Identification of Actinomycetes From Soil and Evaluation of Their Efficiency in Control of the Pathogen Botrytis cinerea Caused Gray Rot Disease on Eggplant,” IOP Conf. Ser. Earth Environ. Sci., vol. 1060, no. 1, p. 012108, 2022, doi: 10.1088/1755-1315/1060/1/012108.
- Valli, S. S. Sugasini, O. S. Aysha, P. Nirmala, P. Vinoth Kumar, and A. Reena, “Antimicrobial potential of actinomycetes species isolated from marine environment,” Asian Pac. J. Trop. Biomed., vol. 2, no. 6, pp. 469–473, 2012, doi: 10.1016/S2221-1691(12)60078-1.
- H. Patel and K. Panchal, “Production and partial purification of pectinase from Streptomyces chartreusis,” Crop Res., vol. 56, no. 1&2, 2021, doi: 10.31830/2454-1761.2021.012.
- Selvin et al., “Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08,” Appl. Microbiol. Biotechnol., vol. 83, no. 3, pp. 435–445, 2009, doi: 10.1007/s00253-009-1878-y.
- Gebreyohannes, F. Moges, S. Sahile, and N. Raja, “Isolation and characterization of potential antibiotic producing actinomycetes from water and sediments of Lake Tana, Ethiopia,” Asian Pac. J. Trop. Biomed., vol. 3, no. 6, pp. 426–435, 2013, doi: 10.1016/S2221-1691(13)60092-1.
- Chithrashree, A. C. Udayashankar, S. Chandra Nayaka, M. S. Reddy, and C. Srinivas, “Plant growth-promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae,” Control, vol. 59, no. 2, pp. 114–122, 2011, doi: 10.1016/j.biocontrol.2011.06.010.
- El Semary, H. Al Naim, and M. F. Aldayel, “A Novel Application of Laser in Biocontrol of Plant Pathogenic Bacteria,” Appl. Sci., vol. 12, no. 10, 2022, doi: 10.3390/app12104933.
- “Preservation Methods of Xanthomonas campestris pv. oryzae in Relation to Virulence and Colony-Type Variation.pdf.”
- D. Makut, K. K. Madaiki, and O. S. Obiekezie, “Molecular characterization of xanthan gum producing Xanthomonas Campestris isolated from dark rot spotted leaves in Keffi, Nasarawa State, Nigeria,” AROC Pharm. Biotechnol., vol. 2, no. 1, pp. 01–08, 2022, doi: 10.53858/arocpb02010108.
- E. Dubrow and A. J. Bogdanove, “Genomic insights advance the fight against black rot of crucifers,” J. Gen. Plant Pathol., vol. 87, no. 3, pp. 127–136, 2021, doi: 10.1007/s10327-021-00987-x.
- G. Patil, C. V. Ambadkar, K. M. Kanase, and V. S. Kashid, “Cultural and Morphological Characteristics of Different Xanthomonas axonopodis pv. punicae Isolates on Nutrient Agar Media,” Int. J. Curr. Microbiol. Appl. Sci., vol. 6, no. 11, pp. 1678–1683, 2017, doi: 10.20546/ijcmas.2017.611.201.
- Velho-Pereira and N. M. Kamat, “Antimicrobial screening of actinobacteria using a modified cross-streak method,” Indian J. Pharm. Sci., vol. 73, no. 2, pp. 223–228, 2011, doi: 10.4103/0250-474X.91566.
- A, “Screening of Antimicrobial Activity and Polyketide Synthase Gene Identification from the Actinomycetes Isolates,” J. Microb. Biochem. Technol., vol. 10, no. 4, 2018, doi: 10.4172/1948-5948.1000404.
- K. Duddu and G. Guntuku, “Isolation, screening and characterization of antibiotic producing actinomycetes from kapuluppada plastic waste dumping yard, visakhapatnam,” Int. J. Pharm. Pharm. Sci., vol. 8, no. 11, pp. 221–229, 2016, doi: 10.22159/ijpps.2016v8i11.10110.
- I. Nabila and K. Kannabiran, “Antagonistic activity of terrestrial streptomyces sp. Vitnk9 against gram negative bacterial pathogens affecting the fish and shellfish in aquaculture,” Rev. Biol. Mar. Oceanogr., vol. 53, no. 2, pp. 171–183, 2018, doi: 10.22370/rbmo.2018.53.2.1291.
- S. Kumar, V. Duraipandiyan, and S. Ignacimuthu, “Isolation, screening and partial purification of antimicrobial antibiotics from soil Streptomyces sp. SCA 7,” Kaohsiung J. Med. Sci., vol. 30, no. 9, pp. 435–446, 2014, doi: 10.1016/j.kjms.2014.05.006.
- M. Shah et al., “Antimicrobial investigation of selected soil actinomycetes isolated from unexplored regions of Kashmir Himalayas, India,” Microb. Pathog., vol. 110, pp. 93–99, 2017, doi: 10.1016/j.micpath.2017.06.017.
- Pathalam et al., “Isolation and molecular characterization of actinomycetes with antimicrobial and mosquito larvicidal properties,” Beni-Suef Univ. J. Basic Appl. Sci., vol. 6, no. 2, pp. 209–217, 2017, doi: 10.1016/j.bjbas.2017.04.002.
- T. Odumosu, O. M. Buraimoh, C. J. Okeke, J. O. Ogah, and F. C. Michel, “ Antimicrobial activities of the Streptomyces ceolicolor strain AOB KF977550 isolated from a tropical estuary ,” J. Taibah Univ. Sci., vol. 11, no. 6, pp. 836–841, 2017, doi: 10.1016/j.jtusci.2017.01.006.
- Elnady, N. M. Sorour, and R. N. Abbas, “Characterization, cytotoxicity, and genotoxicity properties of novel biomediated nanosized-silver by Egyptian Streptomyces roseolus for safe antimicrobial applications,” World J. Microbiol. Biotechnol., vol. 38, no. 3, pp. 1–17, 2022, doi: 10.1007/s11274-022-03231-6.
- Factories, A. R. Ali, Y. Bahrami, E. Kakaei, S. Mohammadzadeh, and S. Bouk, “Isolation and identification of endophytic actinobacteria from Citrullus colocynthis ( L .) Schrad and their antibacterial properties,” Microb. Cell Fact., pp. 1–17, 2022, doi: 10.1186/s12934-022-01936-9.
- Huang et al., “Phenotypic and genomic characteristics of Brevibacterium zhoupengii sp. nov., a novel halotolerant actinomycete isolated from bat feces,” J. Microbiol., vol. 60, no. 10, pp. 977–985, 2022, doi: 10.1007/s12275-022-2134-8.

This work is licensed under a Creative Commons Attribution 4.0 International License.





