How to Cite | Publication History | PlumX Article Matrix
Breaking Barriers with Nanosuspension: A Comprehensive Review
H. K. Malgundkar* , M. D. Pomaje
, M. D. Pomaje and L. S. Nemade
and L. S. Nemade
Govindrao Nikam College of Pharmacy, Sawarde Maharashtra, India.
Corresponding Author E-mail: halima.malgundkar36@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3202
ABSTRACT: Nanosuspension have emerged as a promising and versatile nanotechnology-based approach for improving the solubility and bioavailability of poorly water-soluble drugs. This review article provides a comprehensive overview of the formulation, characterization and application of nanosuspension in pharmaceuticals. The article includes basic principles of nanosuspension preparation, encompassing both top-down and bottom-up methods. It delves into the selection of stabilizers as nanosuspension are sterically stabilised by steric polymers like polyethylene glycol (PEG) and other numerous stabilizers which results into product having particle size in nanometre ranges. Post processing treatment like freeze drying and spray drying can be use to impart stability (thermolabile drugs) in nanosuspension. Different characterization techniques such as electron microscopy, zeta potential, particle size analysis, x-ray diffraction essential for evaluating nanosuspensions have been discussed in this review article. Various advantages of nanosuspension have enabled its use in different dosage forms. The main body of this review focuses on the diverse applications of nanosuspensions including drug delivery, drug targeting and sustained release. Site specific delivery of poorly water-soluble drug is an emerging application of nanosuspension.
KEYWORDS: Homogenization; Microemulsion template; Nanosuspensions; Nanopure; Nanojet; Nanoedge; Precipitation
Download this article as:| Copy the following to cite this article: Malgundkar H. K, Pomaje M. D, Nemade L. S. Breaking Barriers with Nanosuspension: A Comprehensive Review. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Malgundkar H. K, Pomaje M. D, Nemade L. S. Breaking Barriers with Nanosuspension: A Comprehensive Review. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/3unRa7A |
Introduction
Medicines serve as a means of treating and preventing many diseases and disorders, and it is regarded by the global community as a beacon of hope. To ensure the sustainability of the relationship between pharmaceutical researchers and patients, it is imperative to offer a viable and efficacious approach to addressing bodily imbalances. Currently, a wide array of pharmaceutical products is readily accessible in the global market, playing a crucial role in preserving and enhancing the lives of numerous individuals worldwide. In order to achieve the intended therapeutic impact, it is imperative that all drugs adhere to a defined set of criteria and undergo a systematic procedure1. To achieve the intended outcome, it is necessary for the medicine to possess water solubility, enabling its entry into the systemic circulation and subsequent exertion of its therapeutic effects at the targeted region. However, around 50% of the newly developed medications now on the market have lipophilic characteristics and demonstrate limited solubility in water2. The medications in question exhibit suboptimal dissolving profiles and fail to provide the anticipated therapeutic effects due to their restricted solubility in water3. To address this issue, researchers have created various drug delivery systems such as liposomes, noisome, microspheres, nanoparticles, and aquasomes. Among these systems, nanosuspension has emerged as a particularly promising option. A pharmaceutical nanosuspension is characterised as a suspension of solid drug particles that are finely dispersed in an aqueous medium, with the purpose of being administered by various routes such as oral, topical, parenteral, and pulmonary. These suspensions are stabilised by surfactants to ensure their stability and efficacy. The process of nanosuspension entails the production of a formulation that exhibits a decreased particle size, resulting in an enhanced dissolving rate and hence better bioavailability. The solid particles in nanosuspensions typically exhibit a particle size distribution that is generally below one micron, with an average particle size falling within the range of 200 to 600 nm4. The enhancement of the dissolving rate of micronized particles, characterised by a particle size smaller than 10 μm, is directly associated with an augmentation in the surface area, hence leading to an escalation in the dissolution velocity. The dissolving velocity and saturation solubility can be enhanced by the presence of nanoparticles of nano size dimensions, owing to the influence of the vapour pressure effect5. One of the key principles underlying the creation of nanosuspensions is the reduction of bigger particle sizes of lipophilic drugs using various procedures. This reduction is necessary due to the poor solubility and dissolution profile shown by larger particle sizes. The enhancement of aqueous solubility, dissolution profile, and bioavailability of weakly water-soluble drugs can be accomplished by the appropriate use of stabilisers during particle size reduction. The dissolving rate of a drug is influenced by the particle size, with larger particles having a reduced surface area accessible for disintegration, whereas smaller particles boost the dissolution rate by increasing the surface area6. The correlation between the rate of dissolution and the surface area is exactly proportional, as shown by the Noyes-Whitney equation.
The rate of change of x with respect to t may be expressed as dx/dt = AD/h (Cs – Xd/V).
The symbol dx/dt represents the dissolving velocity.
The diffusion coefficient, denoted as D, is a parameter used to quantify the rate at which particles or molecules disperse through a medium.
The variable A represents the surface area of a particle that is in contact with the dissolving media.
The variable “h” represents the thickness of the diffusion layer.
The variable “Cs” represents the saturation solubility of the solute.
The variable Xd represents the concentration of the solute in the medium at a given time t.
The variable V represents the volume of the dissolving media.
Surfactants and stabilisers play a pivotal part in the development process of nanosuspensions. Surfactants have a crucial role in diminishing the interfacial tension between two phases and facilitating optimal dispersion. Surfactants serve not only as suspending agents but also as wetting agents. The optimal concentration of surfactants reduces the surface free energy by reducing the interfacial tension between the solid and liquid mediums. The use of polymers and ionic surfactants as stabilisers during the manufacture of nanosuspensions facilitates the occurrence of electrostatic and steric repulsion. The absence of particle settling in nanosuspensions can be attributed to the phenomenon of Brownian motion, resulting in enhanced physical stability. A system is thermodynamically stable when it exhibits a low surface free energy (∆G).
∆G= γS/L ∆A
The interfacial tension between a solid and a liquid is denoted as γS/L.
The use of nanosuspensions offers several advantages.
The nanosuspension approach is employed for medications that have limited solubility in water. The use of this substance is applicable in the context of targeted drug delivery systems, and its administration can be facilitated through several routes. The delivery of medication by the oral route has several advantages, including a quick beginning of action, a decreased ratio between the fed and fasting states, and an enhanced bioavailability. The solubility of a substance is enhanced by reducing the particle size, as this leads to an increase in accessible surface area. The enhancement of dissolution rate can be achieved by increasing the solubility of lipophilic drugs. The enhancement of absorption is directly correlated with an increase in dissolution rate, resulting in an increase in bioavailability.
Post-production techniques, such as freeze drying, offer extended physical stability to thermolabile pharmaceuticals, ensuring their long-term preservation7.
Disadvantages
Sedimentation and compaction pose significant challenges in relation to nanosuspensions. It is important to use caution when engaging in the processes of handling and transporting.
The attainment of a consistent and precise dosage is unattainable7.
The selection of a drug for nanosuspension preparation is based on specific criteria. Nanosuspensions are suitable for active pharmaceutical ingredients (APIs) that include one or more of the following characteristics8:
A medicine that exhibits high lipophilicity or insolubility in both water and oil may be considered a potential option for nanosuspension formulation, particularly for pharmaceuticals classified under Biopharmaceutics Classification System (BCS) class II and class IV.
Pharmaceutical substances exhibiting reduced solubility of their crystalline form, irrespective of the specific solvent.
An application programming interface (API) that incorporates a significantly high dosage.
Consideration of Formulation In this discussion, we will examine the importance of formulation in academic writing.
Stabilizers
The presence of nano-sized particles with high surface energy may lead to the agglomeration or aggregation of drug crystals9. There are two types of stabilization methods such as electrostatic stabilization and steric stabilization. Electrostatic stabilization usually involves the adsorption of ionic charges on the particle surface resulting in partial repulsion between particles whereas, steric stabilization involves the attachment of non-ionic polymers which leads to repulsion between particles10. Therefore, stabilizers are used to provide effective wetting of drug particles, therefore preventing Ostwald’s ripening and agglomeration, resulting in the formation of physically stable nanosuspensions11,12.
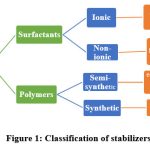 |
Figure 1: Classification of stabilizers. |
Solvents
Organic solvents, such as ethanol, ethyl formate, ethyl acetate, and propylene carbonate, are utilised in formulations due to their water miscibility or partial water miscibility. These solvents are preferred due to their lower toxicity potential and the simplicity with which they may be removed from the formulation13.
Surfactants
Surfactants are known to induce a decrease in interfacial tension, hence enhancing dispersion. In addition, they possess the capability to function as wetting and deflocculating agents. Tween and spans are often employed surfactants in the creation of nanosuspensions14.
Co-surfactants
The selection of a co-surfactant plays a crucial role in the formulation of nanosuspensions employing microemulsions. The phase behaviour can be significantly influenced by the presence of a co-surfactant. Bile salts, dipotassium glycyrrhizinate, and different solubilizers, including Transcutol, glycofurol, ethanol, and isopropanol, have been identified as suitable co-surfactants for incorporation into the formulation, ensuring their safe use15.
Additional Additives
Nanosuspensions may include supplementary substances such as buffers, salts, polyols, osmogents, and cryoprotectants, which are chosen based on the route of administration or the characteristics of the therapeutic component16.
Table 1: Examples of Excipients
| EXCIPIENTS | EXAMPLES |
| Stabilizers | Tween, span series, povidone, cellulosic, poloxamers and lecithin etc. |
| Solvents | Water, methanol, ethanol, chloroform, ethyl acetate, ethyl formate, benzyl alcohol etc. |
| Surfactants and co surfactants | Sodium lauryl sulphate, polyethylene glycol 4000, polysorbate 80, alpha-tocopherol, poloxamer series, glycofurol, transcutol etc. |
| Other additives | Buffer salts, cryoprotectant such as sucrose, lactose, mannitol etc. |
This study explores several techniques employed in the manufacture of nanosuspensions.
There exist two distinct methodologies for the preparation of nanosuspensions, namely Bottom-up technology and Top-down technology.
The bottom-up approach to technology implementation entails the utilisation of uncomplicated and cost-effective equipment. The medication is dissolved in an organic solvent and subsequently precipitated upon the introduction of an anti-solvent in this particular methodology. The process of crystal development is regulated by the incorporation of surfactants. This particular methodology yields a greater degree of saturation solubility in comparison to alternative methodologies17. Several bottom-up processes used for the manufacture of nanosuspensions include the anti-solvent precipitation technique, supercritical fluid process, spray drying, and emulsion-solvent evaporation18. The medication must possess solubility in at least one solvent, and this solvent must exhibit miscibility with a non-solvent.
The top-down approach to technology implementation entails the use of expensive equipment. This method involves the decrease of particle size in big drug particles by the use of several techniques such as media milling, micro fluidization, and high-pressure homogenization. This technique does not employ any aggressive solvents. The implementation of top-down technology necessitates a substantial amount of energy input. This phenomenon is observed in medicines that have limited solubility in both aqueous and organic solvents18.
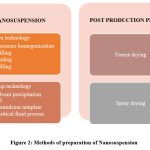 |
Figure 2: Methods of preparation of Nanosuspension. |
Media milling
Media milling is a straightforward and cost-effective technological process. This procedure entails the use of high-shear media mills or pearl mills. The mill is comprised of three main components: a milling chamber, a milling shaft, and a recirculation chamber. The medicine is thereafter introduced into the mill, which is filled with small grinding balls or pearls, in the form of an aqueous solution. The spheres undergo rapid rotational motion at an elevated shear rate inside a carefully regulated temperature environment. The synergistic effects of friction and impact result in a significant decrease in particle size. The milling media, also known as balls, utilised in this study are composed of ceramic-sintered aluminium oxide, zirconium oxide, or strongly cross-linked polystyrene resin, as mentioned in the planetary ball mill research conducted by reference19. In order to obtain effective formulation and to reduce the time of media milling drug can be subjected to prior homogenization followed by media milling20. Nanosuspensions of quercetin, irbesartan, itraconazole are reported by media milling technology.
High pressure homogenization
The process of homogenization entails the passage of the suspension via a valve with a tiny aperture, which is operated under pressure. As the suspension traverses the orifice, the static force diminishes, leading to the vaporization of water and the consequent generation of gas bubbles. When bubbles exit the orifice, they undergo implosion when the suspension returns to normal air pressure, leading to a decrease in particle size. To accomplish particle size reduction in the nanoscale range, it is necessary to undergo many passes or cycles using a homogenizer. The selection of drug hardness, desirable mean particle size, and necessary homogeneity are determining factors in this regard. This technique has the capability to be employed for both diluted and concentrated solutions, facilitating the development of aseptic conditions21. Naringenin nanosuspension prepared by homogenization technique found to be very effective in tumour targeting22. Cyclosporine A nanosuspension was prepared by high pressure homogenization23.
Nanopure
The Nanopure method entails the process of homogenization inside a non-aqueous medium. The drug suspension is subjected to homogenization in medium devoid of water or water mixes at temperatures of 0°C or below, hence earning the term “deepfreeze” homogenization. The findings derived from the implementation of Nanopure technology demonstrate a level of similarity to the outcomes achieved via the use of Dissocube. Consequently, Nanopure technology is considered a promising and developing approach for the manufacture of nanosuspensions. Furthermore, this technology has potential for application in the handling of thermally labile substances under less severe circumstances. Lacidipine nanosuspension was prepared by nano pure method24.
Combined precipitation and homogenization (Nanoedge)
The creation of nanosuspension entails the combined use of precipitation and homogenization, as demonstrated by the Nanoedge process. The fundamental concept behind Nanoedge has similarities to the processes of precipitation and homogenization. The medication is solubilized in an organic solvent, and subsequently, this solution is combined with a compatible anti-solvent to induce precipitation. Due to the limited solubility in a water solvent combination, the medication undergoes precipitation and then undergoes high shear processing. The use of a combination of these strategies yields a reduction in particle size and an improvement in stability within a reduced timeframe. Paclitaxel and prednisolone are reported to be prepared by Nanoedge method25.
Opposite Stream Technology (Nanojet)
The Opposite Stream Technology, also known as Nanojet, involves the division of a suspension stream into many segments, which then collide with one another at high pressure up to 4000 bar at high velocity of 1000m/s to form a colloidal mixture26. The decrease in particle size is attributed to the large shear forces generated during collisions. The manipulation of particle size in this technology is achieved by the regulation of emulsion droplet size.
Supercritical Fluid Method
The Supercritical Fluid Method involves the creation of nanosuspensions from medicinal solutions. Supercritical fluid technology encompasses a range of techniques employed in the manufacturing of nanosuspensions. These methods include the rapid expansion of supercritical solution process (RESS), the supercritical anti-solvent process, and the precipitation with compressed anti-solvent process (PCA). In the process of Rapid Expansion of Supercritical Solutions (RESS), the drug solution undergoes expansion within a supercritical fluid via a nozzle. This expansion causes a decrease in the solvent power of the supercritical fluid, leading to the precipitation of the medication as small particles within the nano size range. The PCA technique involves the atomization of the drug solution within a chamber that contains compressed carbon dioxide (CO2)27. As the solvent is gradually withdrawn, the solution becomes supersaturated, leading to the precipitation of tiny crystals. The supercritical anti-solvent method involves the utilisation of a supercritical fluid, wherein a medication exhibits low solubility, and a solvent that is both miscible with the supercritical fluid and capable of dissolving the drug. The drug solution is introduced into the supercritical fluid, facilitating the extraction of the solvent by the supercritical fluid, and resulting in the drug solution being supersaturated. The substance is then precipitated in the form of small crystals28.
Microemulsion template
The microemulsion template method utilises an organic solvent or a combination of solvents that are impregnated with the medication. These solvents are then dispersed in an aqueous phase that contains appropriate surfactants and stabilisers, resulting in the formation of an emulsion. Subsequently, the organic phase is subjected to evaporation at decreased pressure in order to induce precipitation of drug particles. The internal phase has the potential to consist of either a somewhat miscible solvent or an organic solvent. Di-ultrafiltration is employed to liberate the internal phase and stabilisers of nanosuspensions, facilitating their administration. Microemulsions are dispersions of two immiscible liquids that are thermodynamically stable and exhibit isotropic clarity5.
Precipitation method
The precipitation approach, namely the solvent-antisolvent method, is commonly employed for the synthesis of nanosuspensions. In this methodology, the pharmaceutical compound is initially dissolved in a solvent, followed by the combination of this solution with a miscible antisolvent in the presence of appropriate surfactants and stabilisers. The prompt elucidates that the fast introduction of a drug solution into the antisolvent results in an abrupt increase in drug concentration, leading to the creation of ultrafine crystalline particles typically measuring less than one millimetre in size. The precipitation process encompasses two distinct steps, namely nuclei production and crystal development. In order to create a stable suspension with minimal particle size, it is imperative to have a high nucleation rate coupled with a low growth rate. Both rates are temperature-dependent. According to the methodology employed, it is necessary for the medication to possess solubility in at least one solvent that is capable of mixing uniformly with a non-solvent, hence facilitating the process of precipitation29. Furosemide and pitavastatin nanosuspension was prepared by using nanoprecipitation method30,31.
Dry co-grinding
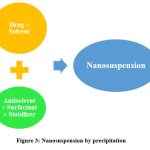 |
Figure 3: Nanosuspension by precipitation |
Recently, the production of Nanosuspensions has been achieved with the implementation of dry milling processes. The enhancement of physicochemical characteristics of pharmaceuticals with low water solubility can be achieved by the process of dry co-grinding. This method leads to an improvement in surface polarity and a transformation of the drug’s crystalline form into an amorphous state. As a result, the solubility profile of the medicine is enhanced. Soluble polymers and copolymers are uniformly distributed inside a liquid medium. Several soluble polymers and co-polymers, including PVP, polyethylene glycol (PEG), hydroxypropyl methylcellulose (HPMC), and cyclodextrin derivatives, have been employed in various applications6. Probucol nanosuspension was prepared by using this method32.
Post-production process
Post-production processing is of utmost importance in ensuring stability of nanosuspension formulations when drug candidates are prone to degradation, such as hydrolytic and chemical degradation, and when stabilisers fail to effectively stabilise the formulation. Different methodologies, such as lyophilization (commonly known as freeze drying) or spray drying, can be employed to generate a dry powder consisting of medication particles at the nanoscale. The conversion of nanosuspension into solid dosage forms is undertaken to enhance patient adherence and ensure optimal stability. Solidification can be accomplished using many procedures, including pelletization, granulation, spray drying, or lyophilization. Silymarin, a herbal medicine known for its hepatoprotective properties, exhibits limited solubility in aqueous environments. To overcome this challenge, it is commonly prepared as a nanosuspension tablet using the lyophilization procedure33. The homogenized nanosuspensions of naringenin were freeze-dried to increase the shelf life of suspension and to study the dissolution behaviour34.
Table 2: Various methods of Nanosuspension’s Drug
| Category | Method | Result | |
| Cefdinir | Antibiotic | Solvent evaporation method | Fast onset of action |
| Clonazepam | Anticonvulsant | Solvent evaporation method | Increased bioavailability |
| Ibuprofen | NSAID | High pressure homogenization | Increases solubility and dissolution |
| Isradipine | Calcium channel blocker | Media milling | Increased solubility and dissolution |
| Carvedilol | Antihypertensive | Antisolvent precipitation | Stable formulation |
| Amphotericin B | Antifungal | Supercritical fluid process | Increased bioavailability |
| Nateglinide | Hypoglycaemic agent | Precipitation method | Increased therapeutic effect |
| Aceclofenac | NSAID | Precipitation method | Fast onset of action |
Characterization of nanosuspensions.
Mean particle size
The mean particle size and particle size distribution are two crucial characteristics that influence the saturation solubility, dissolving rate, physical stability, and in-vivo behaviour of nanosuspensions. Photon correlation spectroscopy (PCS), laser diffraction (LD), and Coulter counter multisizer are commonly employed techniques for the determination of particle size distribution. Particle size distribution (polydispersity index, PI) can also be determined using PCS. The principal investigator (PI) is a crucial factor that dictates the physical stability of nanosuspensions and should ideally be minimised (ranging from 0.1 to 0.25) to ensure the sustained stability of nanosuspensions over an extended period. A PI number within this range suggests a somewhat limited size distribution, but a PI value over 0.5 suggests a significantly wider dispersion6.
Particle charge
The charge of particles, also known as the Zeta potential, plays a crucial role in determining the surface charge characteristics of a nanosuspension. Consequently, it provides valuable insights into the long-term physical stability of the nanosuspension. According to the literature, to achieve electrostatically stabilised nanosuspension, it is recommended to have a minimum zeta potential of ±30mV. On the other hand, for combined steric and electrostatic stabilisation, a minimum zeta potential of ±20mV is required6.
Crystal Morphology
To investigate the impact of high-pressure homogenization on the crystalline structure of the drug, it is possible to employ techniques such as X-ray diffraction analysis in conjunction with either differential scanning calorimetry or differential thermal analysis. X-ray diffraction analysis, in conjunction with differential scanning calorimetry35, may be employed to ascertain both the alteration in the solid state of the drug particles and the magnitude of the amorphous fraction.
Saturation solubility and dissolution rate
It is imperative for the nanosuspension to enhance both the saturation solubility and dissolution velocity. The saturation solubility of a chemical is a constant that is particular to the substance and depends on both the temperature and the parameters of the dissolve media. The rise in saturation solubility may be elucidated using the Kelvin equation and the Ostwald-Freundlich’s equations6.
pH
The pH value of the aqueous formulation should be measured at a certain temperature. The nanosuspension that had been prepared was placed in a 10 mL beaker in order to measure its pH using a pH metre. The addition of electrolyte to the external phase of the formulation for pH stabilisation should be avoided.
Osmolarity
Measurement of Osmolarity in Nanosuspensions using an Osmometer The measurement of osmolarity is a critical factor to consider when preparing a nanosuspension for parenteral delivery. The calculation is theoretically performed using the formula presented in reference 35. The osmolarity may be calculated by dividing the weight in grammes per litre. The product of the number of species, multiplied by 1000, and further multiplied by the molecular weight, is equal to 100.
Surface hydrophilicity and hydrophobicity
Surface hydrophilicity and hydrophobicity are significant factors that influence the dispersion of nanosuspensions inside the body following administration, making them critical criteria to consider. The degree of hydrophobicity shown on the surface plays a crucial role in the initial cellular interactions before phagocytosis. Additionally, it serves as a significant element influencing the adsorption of plasma proteins, which in turn affects the distribution of organs. Hydrophobic interaction chromatography (HIC) is a technology often utilised for the assessment of surface hydrophobicity36.
Adhesion Properties
An in vivo investigation of bioadhesive properties can be conducted using Male Wistar rats as experimental subjects. Typically, each animal is administered a solitary oral dosage of 1ml water solution including 10 mg of drug-loaded nanoparticles (about 45 mg particles/kg body weight). The animal is euthanized by cervical dislocation at 1 and 3 hours after injection. The abdominal cavity is incised, and the stomach, small intestine, and cecum are extracted. These organs are then longitudinally dissected along the mesentery and then washed with a phosphate saline buffer solution at a pH of 7.4. Additionally, the stomach, small intestine, and cecum are divided into segments measuring 2 cm in length. These segments are then subjected to digestion in an appropriate alkaline solution for a duration of 24 hours. The drug was extracted from the digested samples by adding 2 mL of methanol, followed by vortexing for 1 minute and subsequent centrifugation. A 1 ml aliquot of the supernatants is subjected to drug analysis using spectrofluorimetry to determine the proportion of nanoparticle adherence to the mucosa. In order to do calculations, it is also possible to develop a standard curve for the medication7.
Interaction with body proteins
The interaction between nanoparticles and mucin may be examined by in vitro experimentation. This involves incubating mucin and nanoparticles at a weight ratio of 1:4, either in an acidic or neutral solution. The incubation process is conducted with continuous stirring at a temperature of 37°C. The dispersions are subsequently subjected to centrifugation, and a volume of 150μl from each resulting supernatant is aliquoted into a test plate. The Micro BCA Protein Assay Reagent Kit (150μl) was thereafter introduced into the supernatants and the plate, followed by incubation at 37°C for a duration of 2 hours. The absorbance of mucin can be quantified using colorimeters at the wavelength of maximum absorption (λmax) of the medication, as outlined in this approach. The quantity of mucin that adheres to the nanoparticles may be assessed by calculating the disparity between its initial concentration and the concentration seen in the dispersion subsequent to incubation and centrifugation. The calculations can be performed using mucin standard curves as described in reference35.
Table 3: Characterization techniques for Nanosuspension
| Characterization | Instrument/ Method | Standard range/ specification |
| Particle size | Photon correlation spectroscopy Laser diffraction | 0.1- 0.25 |
| Particle charge | Zetameter | ±30mV for stable nanosuspension |
| Crystal morphology | Differential scanning calorimetry, Differential thermal analysis | Broad peak indicates amorphous nature |
| Saturation solubility | Ostwald-Freundlich’s equations | Solubility estimation |
| Compatibility studies | Differential scanning calorimetry | Identification of functional groups |
| pH | pH meter | Required pH can be estimated |
| Dissolution studies | USP apparatus type 1 and type 2 | Maximum percent drug release |
| Polydispersity index | zetasizer | Less than one |
| Interaction with body proteins | Mucin |
Pharmaceutical applications
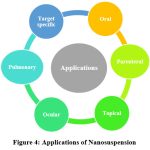 |
Figure 4: Applications of Nanosuspension |
Oral
Oral medication delivery is often regarded as the most desirable form of drug administration. However, drugs classified as BCS class II and class IV have limited solubility in water, which poses challenges for drug dissolution. The nanosuspension technique involves reducing the particle size of the medicine to the nanoscale range, resulting in improved solubility and dissolving rate of the medication. Itraconazole exhibits limited solubility in aqueous environments, necessitating the development of a nanosuspension formulation to enhance its dissolving characteristics and achieve an optimal dissolution profile. The preparation of an Amphotericin B nanosuspension using high pressure homogenization was conducted for the purpose of treating Leishmaniasis37,38.
Parenteral
Parenteral drug delivery is a crucial aspect in emergency situations, such as cardiac arrest and anaphylactic shock, where immediate administration of medication is necessary. Pharmaceutical substances can be administered by several routes, including subcutaneous, intravenous, intramuscular, and intra-arterial administration. The parenteral route of medication delivery circumvents the process of first pass metabolism, leading to enhanced bioavailability of the drug. To prevent the occlusion of capillaries, it is imperative for nanosuspensions supplied via the parenteral route to possess drug particles of a size less than 5µm. Parenteral administration is a viable option for the delivery of anticancer drugs such as paclitaxel nanosuspension. According to a study conducted by researchers, it has been shown that the use of clofazimine nanosuspension exhibits enhanced stability and efficacy39.
Ocular
Certain medications have limited solubility within the lachrymal fluid. The residency duration of a drug in the cul-de-sac can be extended by modifying the surface of nanoparticles with a bio erodible polymer. Additionally, nanoparticles with a positive charge have a robust affinity for mucin with a negative charge, resulting in an extended duration of drug release. Nanosuspensions are utilised to improve the solubility and ocular absorption rate of glucocorticoids, which have limited aqueous solubility40.
Topical
Topical formulations, such as creams and water-free ointments, can be developed by integrating drug nanoparticles into the formulations. The saturation solubility of a medicine in a topical dose form can be increased by using the nano-crystalline form of the molecule. It facilitates the permeation of the medication via the skin.
Pulmonary
Lung drug administration is considered the optimum method for administering medication in illness states such as asthma and chronic obstructive lung disorders. Pulmonary medication administration has the potential to decrease the frequency of dose and provide prompt therapeutic effects at the targeted site of action. The nanosuspension exhibits adherence to mucosal surfaces, hence extending its residence duration. The sustained long-term action of budesonide nanosuspension for pulmonary distribution through nebulizer is achieved using the production process of high pressure homogenization6.
Targeted Drug Delivery
Targeted drug delivery can be employed to administer anti-mycobacterial, fungal, or leishmanial medications specifically to macrophages in cases when the infectious pathogen persists intracellularly. The next course of action for the targeted drug delivery system involves the utilisation of different surface coatings to achieve active or passive targeting. It has been observed that the concentration of atovaquone nanosuspension is significantly elevated in the brain, lungs, sera, and liver. This heightened concentration has resulted in enhanced therapeutic effectiveness against toxoplasma encephalitis in murine models infected with toxoplasma gonidia.
Table 4: Marketed formulations.
| Drug | Indication | Product | Company |
| Sirolimus | Immunosuppressant | RAPAMUNE | Wyethe |
| Fenofibrate | Hypo- cholesteric | Tri- Cor | Abbott |
| Aprepitant | Anti- emetic | EMEND | Merck |
| Megestrol Acetate | Appetite stimulant | MEGACE ER | PAR Pharmaceutical |
Conclusion
This review explores the latest advances in novel drug delivery system and it also assesses the potential of nanosuspension to enhance solubility of poorly water soluble drugs. This article provides a comprehensive analysis of nanosuspension applications in pharmaceutics. The use of post-processing techniques to nanosuspensions enhances their physical stability, enabling their formulation into diverse dosage forms. Nanosuspensions provide broad-ranging uses and have facilitated the implementation of drug delivery systems with site-specific targeting capabilities. Hence, the diverse benefits and potential uses of nanosuspension render this method highly significant from a future-oriented standpoint.
Acknowledgements
I would like to acknowledge my guide Mr. Madan Pomaje and my co guide Ms. Lalita Nemade for their constant guidance and advice to complete this project. I would like to thanks Principal of Govindrao Nikam College of Pharmacy, Sawarde Mr. Anil Battase for encouraging me to complete this work. I would also like to thanks my colleagues, all staff members for directly or indirectly supporting me till the last moment of completion .
I would like to thanks my parents and my husband for supporting and encouraging me and continuous understanding me throughout this project work. I also like to thanks Mr. Mrunal Karanjkar for helping me time to time. Last but not the least, I would like to thank God for blessing me always.
Conflicts of Interest
All the authors confirm that there is no conflict of interest.
Funding Sources
There is no funding sources
Author’ contribution
My co author namely Ms. Lalita Nemade and Mr. Madan Pomaje have guided me regarding this topic and worked as co authors.
Data availability
Not applicable
Ethical Approval
No any studies on human or animal was conducted hence it is not applicable
References
- Shid, R. L.; Dhole, S. N.; Kulkarni, N.; Shid, S. L. Formulation and Evaluation of Nanosuspension Formulation for Drug Delivery of Simvastatin. J. Pharm. Sci. Nanotechnol. 2014, 7 (4), 2650–2665. https://doi.org/10.37285/IJPSN.2014.7.4.7.
CrossRef - Pawar, S.; R.Dahifale, B.; P.Nagargoje, S.; S.Shendge, R. Nanosuspension Technologies for Delivery of Drugs. Nanosci. Nanotechnol. Res. Vol. 4, 2017, Pages 59-66 2017, 4 (2), 59–66. https://doi.org/10.12691/NNR-4-2-4.
CrossRef - Savjani, K. T.; Gajjar, A. K.; Savjani, J. K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 1–10. https://doi.org/10.5402/2012/195727.
CrossRef - (PDF) Nanosuspension: A Promising Drug Delivery System https://www.researchgate.net/ publication/233942587_Nanosuspension_A_Promising_Drug_Delivery_System (accessed Apr 24, 2023).
- Nano-Carrier Systems by Amit K. Goyal, Goutam Rath – Ebook | Scribd https://www.scribd.com/ book/433677289/Nano-Carrier-Systems-Theories-Methods-and-Applications (accessed Apr 24, 2023).
- Yadollahi, R.; Vasilev, K.; Simovic, S. Nanosuspension Technologies for Delivery of Poorly Soluble Drugs. Nanomater. 2015, 2015. https://doi.org/10.1155/2015/216375.
CrossRef - Roshankumar, B.; Nikitha., I.; Sharma, S.; Nishikant, D.; Rishu, T. Nanosuspension: A Review. Rev. J. Pharm. Nanotechnol. 2016.
- View of NANOSUSPENSION: AN OVERVIEW | International Journal of Current Pharmaceutical Research https://journals.innovareacademics.in/index.php/ijcpr/article/view/19584/10961 (accessed Aug 23, 2023).
- Jacob, S.; Nair, A. B.; Shah, J. Emerging Role of Nanosuspensions in Drug Delivery Systems. Res. 2020, 24 (1), 1–16. https://doi.org/10.1186/s40824-020-0184-8.
CrossRef - Li, J.; Wang, Z.; Zhang, H.; Gao, J.; Zheng, A. Progress in the Development of Stabilization Strategies for Nanocrystal Preparations. Drug Deliv. 2021, 28 (1), 19–36. https://doi.org/10.1080/10717544.2020.1856224.
CrossRef - Ahmadi Tehrani, A.; Omranpoor, M. M.; Vatanara, A.; Seyedabadi, M.; Ramezani, V. Formation of Nanosuspensions in Bottom-up Approach: Theories and Optimization. DARU, J. Pharm. Sci. 2019, 27 (1), 451–473. https://doi.org/10.1007/s40199-018-00235-2.
CrossRef - Shetea, G.; Jaina, H.; Punja, D.; Prajapata, H.; Akotiyaa, P.; Bansala, A. K. Stabilizers Used in Nano-Crystal Based Drug Delivery Systems. Excipients Food Chem. 2014, 5 (4), 184–209.
- Ashish Arun Karle; Mrs. Gangotri Yadav; Dr. Ashish Jain; Dr. Bhushan Rane. Nanosuspension Formulation by High Pressure Homogenization (HPH). Int. J. Sci. Res. Sci. Technol. 2022, 115–122. https://doi.org/10.32628/ijsrst229414.
CrossRef - Saddam Hussain, M.; Baquee Ahmed, A.; Debnath, J. Nanosuspension: A Promising Drug Delivery System for Poorly Water Soluble Drug and Enhanced Bioavailability. J. Pharm. Sci. Res. 2020, 11 (10), 4822. https://doi.org/10.13040/IJPSR.0975-8232.11(10).4822-32.
- Nijhu, R. S.; Hossen, F. A Literature Review for Improving the Solubility of Poorly Water-Soluble Drug. 2023, 10 (2).
- Savant, M. Nanosuspension : An Emerging Method of Drug Delivery. 2020, 6 (11), 101–103.
- Aher, S. S.; Malsane, S. T.; Saudagar, R. B. NANOSUSPENSION: AN OVERVIEW. J. Curr. Pharm. Res. 2017, 9 (3), 19–23. https://doi.org/10.22159/IJCPR.2017.V9I3.19584.
CrossRef - Motka, U.; Dabhi, M.; Sheth, N.; Dudhrejiya, A. FORMULATION AND OPTIMIZATION OF NANOSUSPENSION PREPARED BY MEDIA MILLING TECHNIQUE TO ENHANCE THE SOLUBILITY OF ISRADIPINE. J. Pharm. Sci. Drug Res. 2017, 9 (04), 169–177. https://doi.org/10.25004/IJPSDR.2017.090403.
CrossRef - Cheshmehnoor, P.; Bolourchian, N.; Abdollahizad, E.; Derakhshi, A.; Dadashzadeh, S.; Haeri, A. Particle Size Tailoring of Quercetin Nanosuspensions by Wet Media Milling Technique: A Study on Processing and Formulation Parameters. J. Pharm. Res. 2022, 21 (1). https://doi.org/10.5812/ijpr-130626.
CrossRef - Shadab, M.; Alhakamy, N. A.; Akhter, S.; Awan, Z. A. Y.; Aldawsari, H. M.; Alharbi, W. S.; Haque, A.; Choudhury, H.; Sivakumar, P. M. Development of Polymer and Surfactant Based Naringenin Nanosuspension for Improvement of Stability, Antioxidant, and Antitumour Activity. Chem. 2020, 2020. https://doi.org/10.1155/2020/3489393.
CrossRef - Gülbağ Pinar, S.; Çelebi, N. Optimization and Evaluation of Cyclosporine a Nanosuspension Stabilized by Combination Stabilizers Using High Pressure Homogenization Method. Res. Pharm. 2019, 23 (6), 1009–1021. https://doi.org/10.35333/jrp.2019.65.
CrossRef - Panda, S. K.; Dinda, S. C. Development of Lyophilization Cycle for Direct Thrombin Inhibitor and Influence of Excipients and Process Parameter during the Cycle. Curr. Pharm. J. 2014, 3 (5), 259–264. https://doi.org/10.3329/ICPJ.V3I5.18533.
CrossRef - Salazar, J.; Müller, R. H.; Möschwitzer, J. P. Combinative Particle Size Reduction Technologies for the Production of Drug Nanocrystals. Pharm. 2014, 2014, 1–14. https://doi.org/10.1155/2014/265754.
CrossRef - Shinde, M. E.; Maru, A. D.; Mitesh P. Sonawane; Vadje, S. S.; Patil, K. R. Nanosuspension : An Promising Approach To Enhance. World J. Pharm. Res. 2020, 9 (10), 131–146. https://doi.org/10.20959/wjpr202010-18411.
- Misra, S. K.; Pathak, K. Supercritical Fluid Technology for Solubilization of Poorly Water Soluble Drugs via Micro- and Naonosized Particle Generation. ADMET DMPK 2020, 8 (4), 355–374. https://doi.org/10.5599/admet.811.
CrossRef - Pathak, P.; Meziani, M. J.; Desai, T.; Sun, Y. P. Nanosizing Drug Particles in Supercritical Fluid Processing. Am. Chem. Soc. 2004, 126 (35), 10842–10843. https://doi.org/10.1021/JA046914T.
CrossRef - Liu, D.; Xu, H.; Tian, B.; Yuan, K.; Pan, H.; Ma, S.; Yang, X.; Pan, W. Fabrication of Carvedilol Nanosuspensions through the Anti-Solvent Precipitation-Ultrasonication Method for the Improvement of Dissolution Rate and Oral Bioavailability. AAPS PharmSciTech 2012, 13 (1), 295–304. https://doi.org/10.1208/S12249-011-9750-7.
CrossRef - Vadje, S.; K. Surawase, R.; S. Surana, S. Formulation and Evaluation of Nanosuspension Drug Delivery System of Furosemide Produced by Nanoprecipitation Method. Int. J. Pharm. Sci. Rev. Res. 2020, 65 (2), 50–55. https://doi.org/10.47583/ijpsrr.2020.v65i02.009.
CrossRef - Kumar, B. P.; Baig, A. A. Formulation and Evaluation of Pitavastatin Nanosuspension. Chem. Pharm. Sci. 2014, 7 (4), 330–335.
- Li, F.; Li, L.; Wang, S.; Yang, Y.; Li, J.; Liu, D.; Zhang, S.; Wang, S.; Xu, H. Improved Dissolution and Oral Absorption by Co-Grinding Active Drug Probucol and Ternary Stabilizers Mixtures with Planetary Beads-Milling Method. Asian J. Pharm. Sci. 2019, 14 (6), 649–657. https://doi.org/10.1016/j.ajps.2018.12.001.
CrossRef - Chung, N. O.; Lee, M. K.; Lee, J. Mechanism of Freeze-Drying Drug Nanosuspensions. J. Pharm. 2012, 437 (1–2), 42–50. https://doi.org/10.1016/J.IJPHARM.2012.07.068.
CrossRef - Sumathi, R.; Tamizharasi, S.; Sivakumar, T. Formulation and Evaluation of Polymeric Nanosuspension of Naringenin. J. Appl. Pharm. 2017, 9 (6), 60–70. https://doi.org/10.22159/ijap.2017v9i6.21674.
CrossRef - Dahiya, S. 144 An Official Publication of Association of Pharmacy Professionals. Pharm. Res. 2017, 7 (2). https://doi.org/10.21276/bpr.2017.7.2.2.
CrossRef - Patil, O. A.; Patil, I. S.; Mane, R. U.; Randive, D. S.; Bhutkar, M. A.; Bhinge, S. D. Formulation Optimization and Evaluation of Cefdinir Nanosuspension Using 2 3 Factorial Design. 2018. https://doi.org/10.12991/jrp.2018.101.
CrossRef - Ahire, E.; Thakkar, S.; Darshanwad, M.; Misra, M. Parenteral Nanosuspensions: A Brief Review from Solubility Enhancement to More Novel and Specific Applications. Acta Pharm. Sin. B 2018, 8 (5), 733–755. https://doi.org/10.1016/J.APSB.2018.07.011.
CrossRef - Kayser, O.; Olbrich, C.; Yardley, V.; Kiderlen, A. F.; Croft, S. L. Formulation of Amphotericin B as Nanosuspension for Oral Administration. J. Pharm. 2003, 254 (1), 73–75. https://doi.org/10.1016/S0378-5173(02)00686-5.
CrossRef - Wang, X.; Wang, S.; Zhang, Y. Advance of the Application of Nano-Controlled Release System in Ophthalmic Drug Delivery. Drug Deliv. 2016, 23 (8), 2897–2901. https://doi.org/10.3109/10717544.2015.1116025.
CrossRef - prabu, S. L.; Sharavanan, S. P.; Govindaraju, S.; Suriyaprakash, T. N. K. Formulation Development of Aceclofenac Nanosuspension as an Alternative Approach for Improving Drug Delivery of Poorly Soluble Drugs. J. Pharm. Sci. Nanotechnology(IJPSN) 2013, 6 (3), 2145–2153. https://doi.org/10.37285/IJPSN.2013.6.3.6.
CrossRef - Wadhawan, J.; Parmar, P. K.; Bansal, A. K. Nanocrystals for Improved Topical Delivery of Medium Soluble Drug: A Case Study of Acyclovir. Drug Deliv. Sci. Technol. 2021, 65, 102662. https://doi.org/10.1016/J.JDDST.2021.102662.
CrossRef - Patil, O. A.; Patil, I. S.; Mane, R. U.; Randive, D. S.; Bhutkar, M. A.; Bhinge, S. D. Formulation Optimization and Evaluation of Cefdinir Nanosuspension Using 2 3 Factorial Design. 2018. https://doi.org/10.12991/jrp.2018.101.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





