How to Cite | Publication History | PlumX Article Matrix
Comprehensive Review on COVID-19 associated Mucormycosis: Diagnosis, Types and Treatment
Neha Manish Munot1* , Maheshwari Tulsidas Raut2
, Maheshwari Tulsidas Raut2 ,Ujjwala Yadav Kandekar3
,Ujjwala Yadav Kandekar3 , Neeta Rai4
, Neeta Rai4  and Preeti Vinod Gaikwad5
and Preeti Vinod Gaikwad5
1Rajmata Jijau Shikshan Prasarak Mandal’s College of Pharmacy, Pune, India.
2Department of Pharmaceutics, Smt. Kashibai Navale College of Pharmacy, Pune, India.
3Department of Pharmaceutics, JSPMs Rajarshi Shahu College of Pharmacy and Research, Pune, India.
4Department of Pharmaceutics, School of Pharmacy, Vishwakarma University, Pune, India.
5Department of Pharmaceutical Chemistry, KJEI’ Trinity College of Pharmacy, Pune, Maharashtra, India.
Corresponding Author E-mail: nehamunot@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/3227
ABSTRACT: In the past couple of years, the world has been dealing with a dreadful pandemic known as Covid-19 along with one of the life-threatening infection i.e. mucormycosis. Mucormycosis (Zygomycosis) is a fungal infection caused by a group of molds called mucormycosis. Several clinical cases of mucormycosis have been reported globally during the Covid-19 pandemic. Patients with compromised immunity or uncontrolled diabetics or patients that were given steroid therapy to combat infection due to corona virus were at high risk of acquiring mucormycosis as co-morbidity associated with Covid-19. In the present review, various types of mucormycosis, diagnosis, and treatment strategies are explained as it is associated with high morbidity and mortality. Amphotericin B liposomal formulation was found to be the most widely used to treat mucormycosis. Other antifungal agents and adjuvant therapies were also tried to treat this infection.
KEYWORDS: Antifungal drugs; Mucormycosis (zygomycosis); rhino cerebral sinus
Download this article as:| Copy the following to cite this article: Munot N. M, Raut M. T, Kandekar U. Y, Rai N, Gaikwad P. V. Comprehensive Review on COVID-19 associated Mucormycosis: Diagnosis, Types and Treatment. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Munot N. M, Raut M. T, Kandekar U. Y, Rai N, Gaikwad P. V. Comprehensive Review on COVID-19 associated Mucormycosis: Diagnosis, Types and Treatment. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/4a14kXJ |
Introduction
Mucormycosis is a fungal infection that recently affected the individuals suffering from Covid-19. Mucormycosis, commonly known as zygomycosis, is caused by a group of molds known as mucormycetes. It is a fungal infection that is common in immunocompromised patients, including those recovering from COVID-19 or those suffering from cancer or the Human Immunodeficiency Virus (HIV). It is a life-threatening, invasive, opportunistic fungal illness caused by the phylum zygomycete and the rhizopus mucor, absidia, and septate hyphae termed aspergillus, and non-septate hyphae called mucor. Among several Mucormycosis groups, the mucor species and rhizopus species having similar shapes are the most common causative organisms of Mucormycosis. These molds are spread throughout the environment. It causes a lot of swelling and inflammation near the ocular area, and many people may loose their vision as a result of it. While COVID-19-associated pulmonary aspergillosis was becoming more widely known, Mucormycosis was a considerably more serious fungal illness[1, 2]. This fungal infection resulted due to contact with fungal spores in the environment and inhalation of fungal spores fungi in the air.
History of Mucormycosis
Various molds were found responsive for Mucormycosis. These molds are also known as mucormycetes, and they can be found in decaying organic materials such as rotting plants and animal dung. It was first documented in humans in 1885 by Friedeich Kuchenmeister and Fubringer first described the disease in the lungs in 1876. German Paltauf pathologist also recorded the first case in 1885, and rhino-orbital cerebral mucormycosis with diabetes was first reported in 1943 by Harris. The most dangerous complication of covid-19, also known as mucormycosis, was coined by an American pathologist named R. D. Baker [3-5].
Mucormycosis Pathophysiology
Mucormycosis was found in a variety of settings, including damp soil, decomposing organic debris, animal drugs, and leaves. Inhalation was found one of the most prevalent route for spores to reach lungs through our airways. They could be linked to the mucosa of the gut[6]. The following criteria are now used to classify any fungal disease or mycoses.
Site of infection- systemic, cutaneous, superficial and deep or subcutaneous
Acquisition route – exogenous
Virulence- primary or opportunistic.
Rhino-orbital cerebral Mucormycosis
It is important to understand the several Mucormycosis infections. Rhino cerebral Mucormycosis, generally affects the sinus and brain. It was a frequent infection of rhino-orbital-cerebral Mucormycosis that was thought to begin with spore inhalation into the susceptible paranasal sinus. Spores and hyperglycemia, were thought to be the most common conditions in which Mucormycosis developed. Infection was indicated by partial facial swelling, headache, nasal or sinus congestion, black lesions on nose and upper palate of mouth etc. In some incidences, nasal blockage, bloody brown or black nasal discharge and local pain was experienced by the patients. The patients also suffered from facial pain or swelling and headache, orbital pain and loss of the maxillary teeth. The jaw involvement was found to be the classic feature of these forms and serious form of the disease[6, 7].
Pulmonary Mucormycosis
It is infection to lungs indicated by pain in chest, breathlessness, cough, fever etc. Inhalation of spores into the bronchioles and alveoli causes pulmonary Mucormycosis.
In the patients with pneumonia, infraction and necrosis was found common and infection could spread to adjacent structures and disseminate hematogenously to other organs. Hematologic malignancies, and patients on glucocorticoid/deferoxamine therapy or those who had a solid organ transplant were found to be more susceptible to this type of infection[8]. Pulmonary mucormycosis is indicated by symptoms such as fever, cough, chest pain, flexural effusion, hemoptysis, and a worsening of respiratory symptoms[6, 9].
Cutaneous (skin) Mucormycosis
It is infection to skin and indicated by ulcers or blisters on skin and blacking of skin. In addition patient may suffer from discomfort, extreme redness, or swelling surrounding lesion, and patches on the skin. Patients also experienced pain, roughness or extreme redness near the affected area of the skin [10].
Gastrointestinal Mucormycosis
It is the infection to the gastrointestinal region. The symptoms of gastrointestinal mucormycosis are gastrointestinal bleeding, nausea and vomiting, abdominal pain etc.
Poor health and hygienic were found to be most common cause of mucormycosis. These fungi were reported to be non-toxic to most individuals but immunocompromised individuals were found to be most susceptible. Individuals with dug abuse, cancer patients and diabetic patients, particularly diabetic ketoacidosis might be high at risk to this infection.
Disseminated Mucormycosis
It is widespread infection spreading through blood to other organs
Mucormycosis is an infection caused by opportunistic fungus. Other than Covid-19 infection, the risk factors associated with Mucormycosis are presented in the table 1 below:
Table 1: Risk factors for Mucormycosis
| Sr.No | Risk factors | Sr. No | Risk factors |
| 1. | Diabetes Mellitus | 9 | Peritonial dialysis |
| 2. | Autoimmune disorder | 10. | Human Immunodeficiency Virus |
| 3. | Organ transplantation | 11. | Hematologic Malignancy with neutropenia |
| 4. | Malnutrition | 12. | Prior receipt of voriconazole |
| 5. | Burns | 13. | Prior receipt of steroids- corticosteroids |
| 6. | Iron overload | 14. | Diabetic ketoacidosis |
| 7. | Immunosupressive therapy | 15. | natural disasters |
| 8. | Trauma including surgery | 16. | No underlying conditions |
Diagnosis of Mucormycosis
The manifestation of disease of mucormycosis is unspecific and need quickest diagnosis.
Clinically mucormycosis is detected by tissue necrosis as a result of thrombosis and angioinvasion. Many laboratory methods were reported for diagnosis of this infection as listed below:
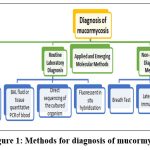 |
Figure 1: Methods for diagnosis of mucormycosis11 |
The Clinical diagnosis includes
Blood tests: Especially neutrophil count
Imaging is very crucial role in identification of fungal species. Reverse halo sign (RHS) on computerized tomography (CT) scan is one more indication of mucormycosis
Positron emission tomography-computed tomography (PET/CT) with [18F]-fluorodeoxyglucose (FDG)
Endoscopic revision
Routine Laboratory Diagnosis
Molecular method
It is processed by methods below
BAL fluid or tissue quantitative PCR of blood
Fluorescent in situ hybridization
Direct sequencing of the cultured organism or formalin-fixed tissue [11]
Histopathology and direct examination of wet mounts as well as cultures help in diagnosis. Biopsy of affected tissues or bronchoalveolar lavage shows presence of fungal hyphae typical for mucormycetes in pulmonary mucormycosis patients. It also confirms the infection is causes by fungus or coinfections with the molds or Aspergillus species. Routine hematoxylin and eosin (H&E) stains, Grocott methenamine-silver (GMS) and periodic acid-Schiff PAS stains, help in a better visualization of the surrounding tissue of fungus.
KOH wet mounts with fluorescent brighteners such as Blankophor and Calcofluor White could be helpful for direct Microscopy for a rapid presumptive diagnosis of mucormycosis. With enhanced visualization of the characteristic fungal hyphae.
Culture of specimens allows identification to the genus and species level during diagnosis of mucormycosis. (MALDI-TOF) is the advanced method of diagnosis cultured Mucorales [12] [13]
Although classical culture and wet mount methods were the standard techniques for detecting Zygomycetes species, recovering organisms from tissues and Bronchoalveolar lavage (BAL) fluid was found to be challenging. Fungal species may not be ample infection causing organisms may not be visible based on representative region of tissue sampled. Furthermore, violent grinding of tissue may cause the coenocytic delicate organisms nonviable. Furthermore, distinguishing Zygomycetes organisms from other filamentous fungi might be difficult [14].
Matrix assisted laser desorption ionization-time of flight mass spectrometry– This method is used for detection of cultured Mucorales [15].
Applied and Emerging Molecular Methods
Confirmation and identification of the infection is done by molecular methods. ITS sequencing is most used and trustworthy method for detection of mucomorcosis. It include several methods like nested PCR, real-time PCR (qPCR), nested PCR combined with RFLP, PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) and PCR/high-resolution melt analysis (HRMA). ITS genomic region targeting with pan-fungal primers is method of choice. qPCR in BAL, also helps in early diagnosis of pulmonary mucormycosis.
Non-Invasive Diagnostic Methods
Due to presence of fungal DNA in the blood qPCR for the detection of circulating mucoralean DNA in blood or urine could be helpful for the diagnosis. Serum Mucorales PCR is a highly trustworthy method for invasive mucormycosis . Probe-based Mucorales-specific real-time PCR assay is rapid and reliable.
Serology ensures availability of antigen markers which can detect Mucorales, as galactomannan (GM) for Aspergillus. The utility of monoclonal antibody (2DA6) in a sandwich ELISA is more with respect to diagnosis. The lateral flow immunoassay (LFIA) for detection of Mucorales has the huge potential of diagnosis of mucormycosis.
Metabolomics-Breath Test was found reliable for breathing profile examination for diagnosis of various species [16].
Micro needle based diagnostics
In this technique the micro needle is used whose length is 1 mm. Various micro needle used for diagnostic purpose for the detection of biomarkers in skin. They are having high potential for diagnosing the diseases. Different material like silicon, metals, polymers, ceramics and glass are used for creating micro needle for the diagnosis purpose [17].
Molecular methods for detecting Mucorales species might improve sensitivity and speed of diagnosis, allowing for earlier and more targeted treatment. The internal transcribed spacer for the detection of zygomycetes in culture have shown molecular detection of zygomycosis accurate [18].
Treatments strategies for mucormycosis
Mucormycosis could be best treated with a multimodal approach such as reversing or stopping influencing factors, primary administration of antifungal medicines, complete excision of infected tissues and adjuvant therapies [19-23].
Corticosteroids and immunosuppressant should be restricted to minimum use during the treatment. Antifungal treatment by using broad-spectrum triazoles and echinocandins had advanced recently, providing highly significant and less harmful substitutes to traditional Polyenes [24, 25]. Nevertheless, because limited numbers of new anti-fungal medicines are currently in progress, the pace of finding is insufficient to meet future requirements. Micafungin was the second echinocandins antifungal drug to be licensed by the FDA in 2005, followed the anidulafungin around 2006 [26]. Triazoles like posaconazole, voriconazole and isavuconazole were also tested to treat this infection [21, 27, 28]. Posaconazole’s was found effective in treatment of difficult-to-treat mucormycosis. Posaconazole was first authorized as an oral solution in 2006, and as a tablet and intravenously in 2013 and 2014[26]. It could be optional drug in patients intolerant to amphotericin B in liposomal formulation. Itraconazole and terbinafine have some effectiveness against some strains. Isavuconazole was a newly developed triazole with antifungal action against Mucorales and other fungal species[29]. Isavuconazole was approved by the FDA in March 2015 [30]. Study of isavuconazole was carried out in a multicenter trial on twenty one patients of mucormycosis and comparison done against standard liposomal formulation of amphotericin B. Patients received 200 mg of isavuconazole daily and after six doses it was found that the isavuconazole was also effective against rare fungal diseases[31]. Hence isavuconazole was suggested as a primary drug for treating mucormycosis[32]. Oral formulations of posaconazole and isavuconazole, were suggested as these could be used for several months [27]. Certain trials were conducted to check the effectiveness of combination of antifungal drugs. Patients treated with a combination of amphotericin B and caspofungin showed positive effects in patients with rhino-orbital- cerebral mucormycosis [33]. Patients treated with an iron-chelator, deferasirox in combination with a polyene had found to be a higher chance of survival in preclinical studies [34]. However, those who received deferasirox had a higher mortality rate in a randomised clinical trial in patients with hematologic malignancies [35]. Other adjuvant therapies such as hyperbaric oxygen and the cytokines injection with antifungal drugs could be preferred. In-vitro and preclinical evidence suggestive of granulocyte-macrophage colony stimulating factor and/or interferon- might promote the immune response towards specific Mucorales [36]. However, there was no clinical data reported for this application, hence these treatments should be used with caution.
Based on the current state of resistant bacteria and the scarcity of novel medications, nanoparticles appear to play a role treat many diseases, especially mycoses fungal infection [37]. The advantages of nanoparticles are boosting the therapeutic efficacy of medications by increasing effectiveness, lowering tolerability, reducing resistance and targeting to specific tissue. The effectiveness of particles as drug carriers was determined by their physicochemical shape, structure, volume, surface composition, route of administration, sustained release, and immune response reactivity [38-42]. Antifungal agents embedded in nanoparticles were shown to be effective in fighting yeast infections in numerous investigations [43, 44]. Fatma et al. (2023) reported the effectiveness of chitosan nanoparticles against M. cirecinelloides [45]. Metallic nanoparticles are gaining considerable attention to treat various fungal infections. Taneja et al. (2023) developed silver nanoparticles to treat rhino orbital mucormycosis. They concluded that silver nanoparticulate were effective to counteract invasive mycosis by interacting with fungal cell membrane [46]. Silver nanoparticles encapsulated in β-cyclodextrin caused reduction in M. ramosissimus proliferation [47]. Pseudomonas indica-mediated silver nanoparticles was also reported to counteract mucormycosis more safely [48]. Fortunately, silver nanoparticle was found effective in few mucor species, still more exploration on other mucor species need to be studied. Nanoparticles of zirconium oxide and zinc oxide has also been emerged to contend against mucormycosis species [49, 50].
To decrease tolerability, lipids in combination with an antibacterial medicine were used [51, 52]. Amphotericin B is prime agent to treat mucormycosis but its application is restricted owing to numerous side effects. It is first line treatment for mucormycosis. It binds with ergosterol of fungal cell membrane and creates pores within it. Complexing amphotericin B with liposomes have better therapeutic index than amphotericin. Amphotericin B liposomes was suggested at a dose of 5 mg/kg/day for infections of central nervous system and 10 mg/kg/day for the infections of peripheral nervous system [53]. High minimum inhibitory concentration of amphotericin B contributed to higher per kg dose to eradicate fungal infection. Three lipid preparations of amphotericin B, amphotericin B lipid complex (ABLC), Amphotericin B colloidal dispersion (ABCD), and liposomal amphotericin B (L-AmB) were studied. ABLC was the first liposome formulation prepared by combining amphotericin B with a lipid in 1:1 ratio. dimyristoyl phosphatidylcholine and l—dimyristoyl phosphatidylglycerol were selected as lipid carrier. ABLC was appeared as ribbon-like formations, size ranged between 1.6 nm to 11.1 nm [54]. Clinical evidences suggestive of reduction in nephrotoxicity as compared with amphotericin B but it was more nephrotoxic than LAmB. ABCL also showed synergistic effect in combination with echinocandins [55]. ABCD is amphotericin B and sodium cholesteryl sulfate arranged in bilayer in 1:1 molar ratio. Sodium cholesteryl sulfate and amphotericin B generates tetramer with hydrophobic and hydrophilic parts in a non-covalent interaction. These spindle fibres form a hard-drive shape of a width of 122 nm and a height of 4 nm [56]. ABCD was reported to decreases the accessibility of amphotericin B in the kidney contributing to reduction in renal toxicity, [57, 58]. Liposomal amphotericin B is unilamellar structure constituting hydrogenated soy phosphatidylcholine forming majority of bilayer, distearoylphosphatidyl glycerol and cholesterol. Distearoylphosphatidyl glycerol exhibit net negative charge and it forms ionic complex with positively charged amphotericin B under acidic condition. Cholesterol also binds with amphotericin B. Thus both these components enhance entrapment of drug in vesicles. LAmB binds to fungal cell wall and undergoes disruption thereby releasing amphotericin B to exert fungicidal activity. It can penetrate in central nervous system and has longer half-life. It is least nephrotoxic than all lipid formulations of amphotericin B [59]. These compositions have a wide range of lipid content and structural properties, as well as distinct pharmacokinetic profiles.
Conclusion
Mucormycosis is a rare fungal disease and majorly affecting immunity compromised individuals. It is a life threatening infection and majorly came into focus during Covid-19 pandemic, where patients suffered from Covid-19 were found to be infected with mucormycosis. This infection can be developed by inhalation or via invasion into skin. Different types of mucormycosis have been identified that affects lungs, nose, mouth skin etc. various laboratory diagnostic methods have been used to identify infection. Still the diagnostic methods needs to be more precise for the detection of this infections. Various antifungal agents have been tried to treat mucormycosis out of which Amphotericin B liposomal formulation was found effective and certain other antifungals such as posaconazole and isavuconazole are under the development phase. There is great need to explore new antifungal drugs at faster and cheaper rate for the treatment of mucormycosis.
Conflict of Interest
All authors declared no conflict of interest.
Funding Source
There is no funding Sources
References
- Binder U., Maurer E. and Lass‐Flörl C. Mucormycosis–from the pathogens to the disease. Clin. Microbiol. Infect., 2014; 20 60-66.
CrossRef - Suganya R., Malathi N., Karthikeyan V. and Janagaraj V. D. Mucormycosis: a brief review. J. Pure Appl. Microbiol., 2019; 13 (1): 161-165.
CrossRef - Brown J. Zygomycosis: an emerging fungal infection. Am. J. Health-Syst. Pharm., 2005; 62 (24): 2593-2596.
CrossRef - Lass‐Flörl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses, 2009; 52 (3): 197-205.
CrossRef - Saegeman V., Maertens J., Ectors N., Meersseman W. and Lagrou K. Epidemiology of mucormycosis: review of 18 cases in a tertiary care hospital. Med. Mycol. J., 2010; 48 (2): 245-254.
CrossRef - Alqarihi A., Gebremariam T., Gu Y., Swidergall M., Alkhazraji S., Soliman S. S., Bruno V. M., Edwards Jr J. E., Filler S. G. and Uppuluri P. GRP78 and integrins play different roles in host cell invasion during mucormycosis. MBio., 2020; 11 (3): e01087-01020.
CrossRef - Gebremariam T., Liu M., Luo G., Bruno V., Phan Q. T., Waring A. J., Edwards J. E., Filler S. G., Yeaman M. R. and Ibrahim A. S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig., 2014; 124 (1): 237-250.
CrossRef - Ben-Ami R., Luna M., Lewis R. E., Walsh T. J. and Kontoyiannis D. P. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J. Infect., 2009; 59 (2): 134-138.
CrossRef - Gebremariam T., Lin L., Liu M., Kontoyiannis D. P., French S., Edwards J. E., Filler S. G. and Ibrahim A. S. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Investig., 2016; 126 (6): 2280-2294.
CrossRef - Castrejón-Pérez A. D., Welsh E. C., Miranda I., Ocampo-Candiani J. and Welsh O. Cutaneous mucormycosis. An. Bras. Dermatol., 2017; 92 304-311.
CrossRef - Bialek R., Konrad F., Kern J., Aepinus C., Cecenas L., Gonzalez G., Just-Nübling G., Willinger B., Presterl E. and Lass-Flörl C. PCR based identification and discrimination of agents of mucormycosis and aspergillosis in paraffin wax embedded tissue. J. Clin. Pathol., 2005; 58 (11): 1180-1184.
CrossRef - Gupta M. K., Kumar N., Dhameja N., Sharma A. and Tilak R. Laboratory diagnosis of mucormycosis: Present perspective. J. Family Med. Prim. Care, 2022; 11 (5): 1664.
CrossRef - McDermott N. E., Barrett J., Hipp J., Merino M. J., Lee C.-C. R., Waterman P., Domingo D. L. and Walsh T. J. Successful treatment of periodontal mucormycosis: report of a case and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 2010; 109 (3): e64-e69.
CrossRef - Dannaoui E. Molecular tools for identification of Zygomycetes and the diagnosis of zygomycosis. Clin. Microbiol. Infect., 2009; 15 66-70.
CrossRef - Cassagne C., Ranque S., Normand A.-C., Fourquet P., Thiebault S., Planard C., Hendrickx M. and Piarroux R. Mould routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS One, 2011; 6 (12): e28425.
CrossRef - Skiada A., Pavleas I. and Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J. Fungi., 2020; 6 (4): 265.
CrossRef - Ponnaiyan D., Anitha C., Prakash P., Subramanian S., Rughwani R. R., Kumar G. and Nandipati S. R. Mucormycosis diagnosis revisited: Current and emerging diagnostic methodologies for the invasive fungal infection. Exp. Ther. Med., 2023; 25 (1): 1-6.
CrossRef - Dannaoui E. Molecular tools for identification of Zygomycetes and the diagnosis of zygomycosis. Clin. Microbiol. Infect., 2009; 15 66-70.
CrossRef - Tissot F., Agrawal S., Pagano L., Petrikkos G., Groll A. H., Skiada A., Lass-Flörl C., Calandra T., Viscoli C. and Herbrecht R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica, 2017; 102 (3): 433.
CrossRef - Gebremariam T., Wiederhold N. P., Fothergill A. W., Garvey E. P., Hoekstra W. J., Schotzinger R. J., Patterson T. F., Filler S. G. and Ibrahim A. S. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob. Agents Chemother., 2015; 59 (12): 7815-7817.
CrossRef - Allen D., Wilson D., Drew R. and Perfect J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti-Infect. Ther., 2015; 13 (6): 787-798.
CrossRef - Amaral A. C., Marques A. F., Muñoz J. E., Bocca A. L., Simioni A. R., Tedesco A. C., Morais P. C., Travassos L. R., Taborda C. P. and Felipe M. S. S. Poly (lactic acid‐glycolic acid) nanoparticles markedly improve immunological protection provided by peptide P10 against murine paracoccidioidomycosis. Br. J. Pharmacol., 2010; 159 (5): 1126-1132.
CrossRef - Chakrabarti A. and Singh S. Management of Mucormycosis. Curr. Fungal Infect. Rep., 2020; 14 (4): 348-360.
CrossRef - Petrikkos G. and Skiada A. Recent advances in antifungal chemotherapy. Int. J. Antimicrob. Agents, 2007; 30 (2): 108-117.
CrossRef - Chen S. C., Playford E. G. and Sorrell T. C. Antifungal therapy in invasive fungal infections. Curr. Opin. Pharmacol., 2010; 10 (5): 522-530.
CrossRef - Chandwani S., Wentworth C., Burke T. A. and Patterson T. F. Utilization and dosage pattern of echinocandins for treatment of fungal infections in US hospital practice. Curr. Med Res. Opin., 2009; 25 (2): 385-393.
CrossRef - Alastruey-Izquierdo A., Castelli M. V., Cuesta I., Monzon A., Cuenca-Estrella M. and Rodriguez-Tudela J. L. Activity of posaconazole and other antifungal agents against Mucorales strains identified by sequencing of internal transcribed spacers. Antimicrob. Agents Chemother., 2009; 53 (4): 1686-1689.
CrossRef - Salas V., Pastor F. J., Calvo E., Sutton D. A., Chander J., Mayayo E., Alvarez E. and Guarro J. Efficacy of posaconazole in a murine model of disseminated infection caused by Apophysomyces variabilis. J. Antimicrob. Chemother., 2012; 67 (7): 1712-1715.
CrossRef - Rybak J. M., Marx K. R., Nishimoto A. T. and Rogers P. D. Isavuconazole: pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy, 2015; 35 (11): 1037-1051.
CrossRef - McCormack P. L. Isavuconazonium: first global approval. Drugs, 2015; 75 (7): 817-822.
CrossRef - Marty F. M., Ostrosky-Zeichner L., Cornely O. A., Mullane K. M., Perfect J. R., Thompson III G. R., Alangaden G. J., Brown J. M., Fredricks D. N. and Heinz W. J. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect. Dis., 2016; 16 (7): 828-837.
CrossRef - Roilides E. and Antachopoulos C. Isavuconazole: an azole active against mucormycosis. Lancet Infect. Dis., 2016; 16 (7): 761-762.
CrossRef - Reed C., Bryant R., Ibrahim A. S., Edwards Jr J., Filler S. G., Goldberg R. and Spellberg B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis., 2008; 47 (3): 364-371.
CrossRef - Ibrahim A. S., Gebermariam T., Fu Y., Lin L., Husseiny M. I., French S. W., Schwartz J., Skory C. D., Edwards J. E. and Spellberg B. J. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig., 2007; 117 (9): 2649-2657.
CrossRef - Spellberg B., Andes D., Perez M., Anglim A., Bonilla H., Mathisen G. E., Walsh T. J. and Ibrahim A. S. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob. Agents Chemother., 2009; 53 (7): 3122-3125.
CrossRef - Roilides E., Antachopoulos C. and Simitsopoulou M. Pathogenesis and host defence against Mucorales: the role of cytokines and interaction with antifungal drugs. Mycoses, 2014; 57 40-47.
CrossRef - Weissig V., Pettinger T. K. and Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomedicine, 2014; 9 4357–4373.
CrossRef - Couvreur P. Nanoparticles in drug delivery: past, present and future. Adv. Drug Deliv. Rev., 2013; 65 (1): 21-23.
CrossRef - Calixto G., Bernegossi J., Fonseca-Santos B. and Chorilli M. Nanotechnology-based drug delivery systems for treatment of oral cancer: a review. Int. J. Nanomedicine, 2014; 9 3719–3735.
CrossRef - Wilczewska A. Z., Niemirowicz K., Markiewicz K. H. and Car H. Nanoparticles as drug delivery systems. Pharmacol. Rep., 2012; 64 (5): 1020-1037.
CrossRef - Cui J., Yang Y., Zheng M., Liu Y., Xiao Y., Lei B. and Chen W. Facile fabrication of graphene oxide loaded with silver nanoparticles as antifungal materials. Mater. Res. Express, 2014; 1 (4): 045007.
CrossRef - des Rieux A., Fievez V., Garinot M., Schneider Y.-J. and Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J. Control. Release, 2006; 116 (1): 1-27.
CrossRef - Baumgartner J., Bertinetti L., Widdrat M., Hirt A. M. and Faivre D. Formation of magnetite nanoparticles at low temperature: from superparamagnetic to stable single domain particles. PloS. one., 2013; 8 (3): e57070.
CrossRef - Chander J., Stchigel A. M., Alastruey-Izquierdo A., Jayant M., Bala K., Rani H., Handa U., Punia R. S., Dalal U. and Attri A. K. Fungal necrotizing fasciitis, an emerging infectious disease caused by Apophysomyces (Mucorales). Rev. Iberoam. Micol . 2015; 32 (2): 93-98.
CrossRef - Abo El-Ela F. I., Hassan W. H., Amer A. M. and El-Dek S. I. Antifungal Activity of Chitosan Polymeric Nanoparticles and Correlation with Their pH Against Mucor circinelloides Causing Mucormycosis, Along with Penicillium notatum and Aspergillus Species. Curr. Microbiol., 2023; 81 (1): 47.
CrossRef - Chatterjee K., Taneja J., Khullar S. and Pandey A. K. Antifungal activity of silver nanoparticles on fungal isolates from patients of suspected mucormycosis. Int. Microbiol., 2023; 26 (1): 143-147.
CrossRef - George C., Kuriakose S., George S. and Mathew T. Antifungal activity of silver nanoparticle-encapsulated β-cyclodextrin against human opportunistic pathogens. Supramol. Chem., 2011; 23 (8): 593-597.
CrossRef - Salem S. S., Ali O. M., Reyad A. M., Abd-Elsalam K. A. and Hashem A. H. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. J. Fungi., 2022; 8 (2): 126.
CrossRef - Mohamed D. Y. Detection the antifungal effect of zirconium oxide nanoparticles on mold which isolated from domestic’s bathroom. M.J.S., 2018; 29 (1): 15-22.
CrossRef - Zeng X., Zhang F., He N., Zhang B., Liu X. and Li X. ZnO nanoparticles of different shapes and their antimycotic property against penicillium and mucor. Nanosci. Nanotechnol. Lett., 2016; 8 (8): 688-694.
CrossRef - Carrillo-Munoz A., Quindos G., Tur C., Ruesga M., Miranda Y., Valle O. d., Cossum P. and Wallace T. In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J. Antimicrob. Chemother., 1999; 44 (3): 397-401.
CrossRef - Quindós G., Carrillo-Muñoz A., Ruesga M., Alonso-Vargas R., Miranda Y., Tur-Tur C., Rubio M., Wallace T., Cossum P. and Martin-Mazuelos E. In vitro activity of a new liposomal nystatin formulation against opportunistic fungal pathogens. Eur. J. Clin. Microbiol. Infect. Dis., 2000; 19 (8): 645-648.
CrossRef - Benincasa M., Pacor S., Wu W., Prato M., Bianco A. and Gennaro R. Antifungal activity of amphotericin B conjugated to carbon nanotubes. ACS nano., 2011; 5 (1): 199-208.
CrossRef - Hamill R. J. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs, 2013; 73 (9): 919-934.
CrossRef - Reed C., Bryant R., Ibrahim A. S., Edwards Jr J., Filler S. G., Goldberg R. and Spellberg B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis., 2008; 47 (3): 364-371.
CrossRef - Working P. Amphotericin B colloidal dispersion. Chemotherapy, 1999; 45 (Suppl. 1): 15-26.
CrossRef - Groll A. H., Mickiene D., Piscitelli S. C. and Walsh T. J. Distribution of lipid formulations of amphotericin B into bone marrow and fat tissue in rabbits. Antimicrob. Agents Chemother., 2000; 44 (2): 408-410.
CrossRef - Fielding R., Singer A., Wang L., Babbar S. and Guo L. Relationship of pharmacokinetics and drug distribution in tissue to increased safety of amphotericin B colloidal dispersion in dogs. Antimicrob. Agents Chemother., 1992; 36 (2): 299-307.
CrossRef - Stone N. R., Bicanic T., Salim R. and Hope W. Liposomal amphotericin B (AmBisome®): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs, 2016; 76 485-500.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





