How to Cite | Publication History | PlumX Article Matrix
Enhanced Yield of Mungbean (Vigna radiata L.) using Bacterial Biofertilizer
Brijesh Kumar Yadav1 , Nakulananda Mohanty 1
, Nakulananda Mohanty 1 , Satyabrata Dash2
, Satyabrata Dash2 , Shubham Pradhan2
, Shubham Pradhan2 , Bijayananda Sahoo2
, Bijayananda Sahoo2 and Biswajit Rath2*
and Biswajit Rath2*
1Department of Zoology, Maharaja Sriram Chandra Bhanja Deo University, Baripada, Odisha India.
2Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University, Baripada, Odisha India.
Corresponding Author E-mail:brath_2000@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/3205
ABSTRACT: Mungbean (Vigna radiata L.) is a rich source of protein, fibers, minerals and vitamins. Considering the recent sudden increase of interest in the use of grain legumes, the present study was framed to enhance the yield of Mungbean using biofertilizer to improve human nutrition. Thus, a comparative effect of biofertilizers in individual and consortia of microbial inoculants like Rhizobium sp., Pseudomonas putida (PSB) and Frateuria aurantia (KMB) and Organic manure like vermicompost on the growth and yield of Mungbean was studied. Different parameters which include shoot length, biomass and pH was analyzed over a period of 3 months in field trials. Farm yard manure and commercial chemical fertilizers were used as comparative controls. The plants grown with individual biofertilizer of Rhizobium sp. did not show significant results in the morphological and physical parameters. On the contrary, consortium biofertilizers i.e., Rhizobium sp., P. putida and F. aurantia showed observable yield of Mungbean. The results concludes that biofertilizers in different combinations could be a potent eco-friendly measure to boost the Mungbean crop yield, thereby increasing the income of farmers and also improving the availability of pulses in our country.
KEYWORDS: Biofertilizer; crop; legume; mungbean; Rhizobium; yield
Download this article as:| Copy the following to cite this article: Yadav B. K, Mohanty N, Dash S, Pradhan S, Sahoo B, Rath B. Enhanced Yield of Mungbean (Vigna radiata L.) using Bacterial Biofertilizer. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Yadav B. K, Mohanty N, Dash S, Pradhan S, Sahoo B, Rath B. Enhanced Yield of Mungbean (Vigna radiata L.) using Bacterial Biofertilizer. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/4ag3PIM |
Introduction
Vigna radiata L. (Mungbean) is a native Indian crop that is also referred to as green gram or moong. It is an essential food and economic crop in the rice-based farming systems of Southeast Asia1. The mungbean is an immensely important edible legume crop. Legumes are the prominent edible crops with a higher content of essential components like protein, minerals, vitamins which are easy to digest; encourage their consumption by humans for several centuries as an alternative source of animal protein and are known as “the poor man’s meat” 2,3, 4,5. Asian countries, including India, China, Bangladesh, Pakistan, and many Southeast Asian territories, are the major Mungbean-producing countries; however, due to its short growth cycle, it is also cultivated in arid regions of southern Europe, warmer parts of Canada, and USA 6. Mungbean possess diverse range of phytoconstituent such as polyphenols, polysaccharides, and peptides, all of which have been linked to considerable human health benefits. Mungbean may be regarded as an alternate functional food 7,8,9 which is commonly used as a whole grain and valued for its excellent protein content (24-25% DW of the seed) 10. Mungbean is composed of 25–28% protein, 1.0–1.5% fats, 3.5–4.5% fibers, and 60 to 65% carbohydrate and abundant with lysine, ascorbate, potassium, iron (Fe), phosphorus (P), and calcium (Ca), which highlights its nutritional as well as good antioxidant properties 11,12,13. Mungbean has the multidimensional potentiality to India’s national economy for its variety, ease of digestion, palatability, and higher market value 14.
Since Mungbean proved to be a vital nutrient reach herb and is a common household item, thus to feed the growing population attention must be rendered to increase the yield. The crop yield depends substantially on the use of fertilizers. The application of chemical fertilizers has been proven most effective but due to their hazardous effect bioformulation emerges as potential substitutes, as they are inexpensive and nontoxic to agro-ecosystem. The use of plant growth-promoting rhizobacteria (PGPR) to improve crop sustainability is becoming a sustainable agriculture practice 15. The application of plant growth promoting substances (PGPR) in agricultural activities as inoculants is being very demanding since it would significantly reduce the burden on use of chemical fertilizers and pesticides. The gradual use of PGPR gaining acceptance, intended to isolate several species of bacteria and to study their role to promote plant growth. The research outcome on potent PGPR strains with multiple attributes indicated various genera of bacteria (Azotobacter, Pseudomonas, Rhizobium and Bacillus) revealing promising results16.
Materials and Methods
Soil Sampling
Soil samples were collected from the rhizosphere of Vigna radiata (Mungbean) in Sathariya, Jaunpur (25° 44′ 55.3020” N and 82° 41′ 54.3876” E), Uttar Padesh, India. Sampling was done at the mungbean-cultivated field. The sample was collected using a sterile spatula from the surface and subsurface layer of the soil and kept in sterile plastic bags. The soil was processed by removing all extraneous substances.
The soil sample (10g) was suspended in 90 ml of saline buffer and mixed thoroughly by a rotatory shaker for 10min. The soil sample was diluted up to 102 – 106 dilutions with sterile saline buffer. For the isolation of rhizospheric bacteria, nutrient agar media was prepared. Sterilized media were poured aseptically into Petri plates and allowed to solidify. After solidification, the diluted soil suspension was spread uniformly on the plates using a sterile spreader. Then the plates were incubated at 30±1ºC for 24-48 hrs. One day old culture was streaked on Pikovskaya’s medium containing bromophenol blue and incubated for 48hrs at 28±1ºC.
Method of Isolation of Rhizobium
The Rhizobium sp. was segregated and isolated from the root nodules of Vigna radiata (Mungbean) plant. Nodules on the roots were round, 2 to 4 mm in size, and looks pink in colour. Root nodules were sterilized in 95% ethanol and then washed repeatedly with distilled water. Further, the nodules were crushed and streak plated on Yeast Extract Mannitol agar with 0.0025% (w/v) Congo red and incubated at a temperature of 30 o C for 48 hrs to get pure colonies2.
Preparation of seed treatment with inoculums
Seeds were counted and kept in separate polythene bags according to treatment. Seeds were than moistened and mixed thoroughly with bacterial suspension and adjusted to a final density of 108 CFUs/ml. The Mungbean seed was inoculated in broth culture for 30 minutes with 0.1% Carboxymethyl cellulose and thereafter soaked seeds were air dry at shade place.
Biochemical Identification
Gram staining method
Bacterial cultures were grown in suitable medium at a temperature of 37° C for 24 hrs. For staining purpose fresh cultures were used. After incubation period, cultures were shifted aseptically to 1.5 ml Eppendorf tubes and centrifuged for 5 min at 6, 000 rpm. Further, the supernatant was removed and cells were again poured into distilled water. Subsequently Gram staining was performed and under light microscopy Gram positive bacteria were identified, isolated and cultured.
Molecular Identification
Data validation: PCR analysis was carried out by using 22nt of universal primer (forward and reverse) and sequence generate by using Sanger dideoxy sequencing method (Agrigenome, Odisha). All nucleotide are NCBI (National centre for Biotechnology Information)/BLAST (http://www.ncbi.nlm.nih.gov/ Basic Local Alignment Search Tool) analysis and submitted to GenBank with accession of GenBank Rhizobium sp. SuBiCo_MSCBU-2 (PP112100), Pseudomonas sp. SuBiCo_MSCBU-1 (PP112097) and Frateuria sp. SuBiCo_MSCBU-3 (PP112101) (Fig 2 A-C). Proofreads were edited and assembled in MEGA (Molecular Evolutionary Genetics Analysis-Version 11) and Sequencher v. 5.
Field Experimental trial
Bio-efficacy of the liquid-based formulation was evaluated under field conditions (mini plots). Experiment was laid out in randomized complete block design (RCBD) with three replicates. The area of each plot was 4 m2 (2m x 2m) and space between blocks and plots were 0.8 and 1.0 ft, respectively. The distances between lines are 20 cm. All plots consisted of different treatment.
During field experiments, the seeds of Mungbean were treated with individual and consortia of microbial inoculants, in the pattern as: T-0 = Control (Un-inoculated), T-1 = vermicompost, T-2 Vermicompost + Rhizobium sp., T-3 = Vermicompost + KMB, T-4 = Vermicompost + PSB, T-5 = Vermicompost + KMB + PSB + Rhizobium sp. and T-6 = chemical (Urea+Di ammonium phosphate). Summer Mungbean variety Malviya Jankalyani (HUM-16) was used as the experimental crop, the seeds were sown 3 cm deep inside the soil. Farm yard manure and commercial chemical fertilizers were used as comparative controls. The growth of plants was periodically recorded on 30 days and 45 days. The study conducted over a period of 3 months including complete field trials. Thereafter at the end of experiment, biomass of plants was determined by fresh shoot weight of aerial parts of plants and those parts were dried at 60ºC under hot air oven for three days and further dry weight of those parts was recorded. The pH of soil also measured prior to sowing and after harvesting of crop.
Statistical analysis
All the analysis was carried out triplicate investigation, the experimental value interpreted by calculation of average (mean) and standard deviation (SD) using SPSS software for windows release 19.0 version (SPSS Inc., IBM, New York, USA).
Results
Screening of bacterial colonies
The Rhizobium strains were isolated from the root nodules of Vigna radiata and streak plating was done on yeast extract Mannitol agar and Congo red, in which single Rhizobium colonies were selected and streaking was performed on YEM agar to get pure culture (Fig. 1a). For the isolation of phosphate-solubilizing bacteria Pseudomonas putida, Pikovskaya’s medium containing bromophenol blue was used and incubated for 48hrs at 28±1ºC. Soon after the incubation period, yellow-colored clear zones were found around the each bacterial colony. P. putida in response to low pH produced by the release of organic acids enhanced phosphate solubilization (Fig.1b)17. Potassium solubilization ability of bacterial strains F. aurantia was identified by single streaking on Aleksandrov agar medium having di-potassium hydrogen phosphate as the sole source of insoluble inorganic potassium (Fig. 1c). After inoculation, plates were incubated at 28±1ºC for 45-48 hrs, observed for clearing zones around the colonies (indication of solubilization of inorganic potassium by bacteria).
 |
Figure 1: Isolated bacteria (a) Rhizobium sp., (b) Pseudomonas putida (c) F. aurantia |
Molecular Identification
Phylogenetic analysis
 |
Figure 2A: Phylogenetic analysis of Rhizobium sp. by using Maximum Likelihood method of MEGA 11 software |
 |
Figure 2B: Phylogenetic analysis of Pseudomonas sp. by using Maximum Likelihood method of MEGA 11 software |
 |
Figure 2C: Phylogenetic analysis of Frateuria sp. by using Maximum Likelihood method of MEGA 11 software |
Evolutionary relationship of taxa
The evolutionary history of three PGPR bacteria was inferred by using the Maximum Likelihood method and Tamura-Nei model. The tree with the highest log likelihood for Frateuria sp. (-3957.41), Rhizobium sp. (-1861.50) and Pseudomonas sp. (-2156.93) is calculated. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura-Nei model, and then selecting the topology with superior log likelihood value. The proportion of sites where at least 1 unambiguous base is present in at least 1 sequence for each descendent clade is shown next to each internal node in the tree. This analysis involved 10 nucleotide sequences. All positions containing gaps and missing data were eliminated (complete deletion option). There were a total of 1358 positions (Rhizobium sp.), 1513 positions (Pseudomonas sp.) and 1457 positions (Frateuria sp.) in the final dataset. Evolutionary analyses were conducted in MEGA1118.
Morphologically, PGPR bacteria are identified as belonging to the genera Pseudomonas and Frateuria. Molecular studies have further refined this identification, with sequence analysis indicating the closest resemblance to Pseudomonas putida and Frateuria aurantia through BLAST analysis. Subsequently, submissions of both bacterial species to GenBank were labelled as PGPR clones, and they were automatically published by the taxonomic browser under the generic classifications of Pseudomonas sp. and Frateuria sp.
Evaluation of growth and biomass after fertilizer treatment
The present study revealed the impact of inoculation of Rhizobium sp, P. putida and F. aurantia on plant growth promotion. Under field condition significant increase in shoot length and biomass as mentioned in fig. 3 & fig. 5 respectively was observed. However, the use of consortia of microbial inoculants has significant attributes in total yield by applying control (without chemical and bio-fertilizer), inorganic and biofertilizers (fig. 4). Present finding demonstrates the efficacy of Rhizobium sp, Pseudomonas putida and Frateuria aurantia in promoting plant growth and yield which was clearly distinct between inoculated and uninoculated (controls) Mungbean. However, the degree of plant growth enhancement in Mungbean inoculated with isolated strains varies and was significantly different from the uninoculated control. The results presented in Fig. 5 demonstrate the effect of rhizobacteria treatment on fresh biomass of Mungbean plants. Among the PGPRs, rhizobacteria strains significantly improved the fresh shoot biomass of Mungbean compared to their corresponding uninoculated controls. Similarly, inoculation with rhizobacterial strains indicated promising increase in the dry weight of Mungbean compared to uninoculated control (Fig. 5). The study results indicated that all the test rhizobacteria strains helps in increasing the shoot length, dry weight and total yield of Mungbean compared to uninoculated control (Fig. 4 & 5). Promising growth in shoot length was determined in response to inoculation mixture of rhizobacteria i.e., P. putida +Rhizobium sp+ F. aurantia together. Experimental result indicated markable ability of the Rhizobacteria to enhance the shoot length. Field trial on the effect of PGPR inoculation on growth of Mungbean on the study parameters like shoot length (cm), shoot fresh biomass (mg), dry weight (mg) and total yield (g) are marked different according to LSD (p ≤ 0.05) (Fig. 3).
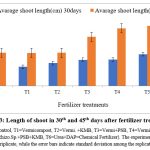 |
Figure 3: Length of shoot in 30th and 45th days after fertilizer treatment |
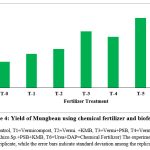 |
Figure 4: Yield of Mungbean using chemical fertilizer and biofertilizer |
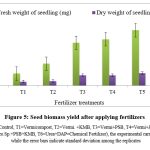 |
Figure 5: Seed biomass yield after applying fertilizers |
Biochemical Estimation
Table 1 Impact of PGPR with varied Nitrogen, Phosphate, and Potassium concentrations on Mungbean grain nutrient content (%) in controlled culture experiments
| Treatments | Yield/plot (g−1) | N% | P% | K% |
| T0 | 98.67±0.33 | 0.89 ± 0.02 | 0.202 ± 0.007 | 0.164 ± 0.005 |
| T1 | 147.09±0.53 | 1.12 ± 0.03 | 0.237 ± 0.004 | 0.177 ± 0.007 |
| T2 | 214.03±0.02 | 1.06 ± 0.024 | 0.231 ± 0.005 | 0.199 ± 0.009 |
| T3 | 193.05±0.16 | 1.04 ± 0.03 | 0.258 ± 0.003 | 0.154 ± 0.003 |
| T4 | 113.02±0.06 | 1.25 ± 0.031 | 0.221 ± 0.002 | 0.181 ± 0.003 |
| T5. | 265.05±0.11 | 1.64 ± 0.027 | 0.283 ± 0.002 | 0.210 ± 0.002 |
| T6 | 129.07±0.19 | 1.22 ± 0.033 | 0.223 ± 0.003 | 0.178 ± 0.007 |
(Where, T0=Control, T1=Vermicompost, T2=Vermi. +KMB, T3=Vermi+PSB, T4=Vermi+Rhizobium Sp., T5=Vermi+Rhizo.Sp.+PSB+KMB, T6=Urea+DAP=Chemical Fertilizer), The values are expressed as mean with standard deviations (±SD)
Soil pH after fertilizer treatment
The fertilizer treatment in the field alters the pH of the soil (Fig. 6). The application of chemical fertilizer (T6) slightly increases the pH, 7.6± 0.88 (before treatment) and 7.83 ± 0.26 (after treatment), but in the treatment of T2 (Vermi. +KMB), the pH slightly decreases. However, administration of T5 (Vermi+Rhizo.Sp.+PSB+KMB) decreases the soil pH to alkaline range i.e., 7.78± 0.78 (before treatment) and 7.56 ± 0.36 (after treatment). The pH alternation induces phosphate, potash solubilization, and nitrogen-fixing bacteria boosting the macronutrient richness and make the medium (soil) alkaline and increases the fertility.
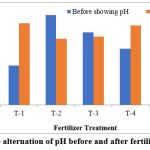 |
Figure 6: The alternation of pH before and after fertilizer treatment |
Discussion
Nitrogen, Phosphorus, and Potassium are important macroelements for plant nutrition, which has immense importance in all physiological and biochemical functions of the crop plant, including photosynthesis, macromolecular biosynthesis, and respiration. Phosphorus can be found in both organic and inorganic forms in soil. Plants are unable to utilize phosphate because 80-85% of it is insoluble, immobilized, or precipitated. Plants can use phosphate in two soluble forms: monobasic i.e H2PO4 and diabasic i.e HPO4 ions19. There are many phosphate solubilizing bacteria (PSB) in the rhizosphere, including Rhizobium sp., Arthrobacter sp., Bacillus sp., Beijerinckia sp., Enterobacter sp., Erwinia sp., Flavobacterium sp., Pseudomonas sp., Rhodococcus sp., and Serratia sp.20,21,22. Similarly, potash (k) solubilizing bacteria (KSB) such as Achromobacter sp., Alcaligenes sp., and Bacillus sp., may be used as potassium bio-inoculants for the reduction of potassium deficient soil at high temperatures 23, 24, 25, which use different strategies to make available for plants so that it can be absorbed. However, nitrogen is abundant in the environment, but N2 accumulation necessitated nitrogen mobilization, which needed urea breakdown or fixation. Several species of Rhizobium sp., Arthrobacter sp., Azospirillum sp., Azotobacter sp., Bacillus sp., Enterobacter sp., Gluconacetobacter sp., Klebsiella sp., Pseudomonas sp., and Serratia sp. isolated from the rhizosphere of various crops and are responsible for nitrogen solubilization and fixation26. The possible inherent mechanisms of PGPR for the solubilization of macronutrients and catalysis of certain compounds rich in minerals, releasing extracellular enzymes, during substrate degradation process.27
The present research focused on isolated Rhizobium sp., P. putida (PSB), and F. aurantia (KMB) from the rhizosphere of Vigna radiata (Mungbean), similar to the work of De Souza et al. 28 who identified Burkholderia sp., Cedecea sp., Cronobacter sp., Enterobacter sp., and Pseudomonas sp. capable of solubilizing tricalcium phosphate from the rhizosphere of rice. Similarly, Yadav and Pandey 29 isolated P-solubilizers from the tomato rhizospheric region, including Bacillus sp., Streptomyces sp., and Cronobacter sp. Further, Verma et al. 23, 24, 25 isolated K-solubilizing bacteria from the Triticum aestivum rhizosphere, including Achromobacter sp., Alcaligenes sp., Bacillus sp, Methylobacterium sp., Pseudomonas sp., Rhodobacter sp., and Salmonella sp.. Qureshi et al. 30 isolated Rhizobium sp. from Vigna radiata L. for research on auxin production and P- solubilization. Rhizobium sp. is well known for producing hormones such as auxins, N2– metabolism, and P-solubilization, which are thought as the most reliable means for enhanced plant growth and yield 31. The data obtained during the present investigation revealed that individual PGPR showed noteworthy result on morphological and physical parameters. On the contrary, the consortium of PGPR like Rhizobium sp., P. putida (PSB) and F. aurantia (KMB) showed most significant yield of Mungbean in T5- type fertilizer (Vermicompost +Rhizobium sp.+ PSB + KMB) application along with other treatment and addition of potassium to the inoculants results efficient growth and biomass yield. From the study of Navsare et al. 32, fertilizer treatment with mixture of all inoculants i.e., T5 (RDF + Rhizobium + PSB + KSB + ZSB) found superior over control and that support to our findings. Besides this, individual biofertilizer F. aurantia (KMB) also showed encouraging result, possibly because of potassium being directly responsible for flowering and fruiting of crop33. However, soil fertility is deteriorating with the passage of time, and Mungbean is facing nutrient deficiency, especially P and K34. Mungbean being a nitrogen-fixing crop has low deficiency of nitrogen except at the initial stage35.
PGPRs has been extensively examined in several plant species to determine the increased crop yield. Plant growth promoting (PGP) activities in several crop plants have been confirmed in multiple bacterial genera of proteobacteria. Three isolated strains (P. putida, Rhizobium sp, and F. aurantia) were evaluated for PGP activity in Mungbean in the current study. The study results indicated that test strains and the fertilizer treatments combindly determined to have the greatest potential for upliftement of Mungbean growth in terms of height, biomass, and total productivity.
Conclusion
The present study concluded a better understanding on the role of bio-inoculants’ as fertilizer where the nutrient uptake increases substantially. The study results also indicated that plants grown with individual biofertilizer Rhizobium sp. provides better expression in the morphological and physical parameters whereas consortium of microbes Rhizobium sp., PSB (P. putida) and KMB (F. aurantia) used as biofertilizers has greater yield of Mungbean. Additionally, F. aurantia used as biofertilizer has more promising response, possibly because potassium is highly responsible for flowering and fruiting in crop and the yield was more than controls. Despite extensive research to date, the inherent potential of PGPRs must be revealed for commercialization and suitable approach for sustainable agriculture, which needs proper formulations, agricultural practice and field trials which together can give sustainable economic prosperity.
Acknowledgment
The authors extend thanks to Head Department of Biotechnology and Zoology, Maharaja Sriram Chandra Bhanja Deo University, Takatpur Baripada, Odisha for providing laboratory facilities for conducting the experiments.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
Authors’ Contribution
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by Brijesh Kumar Yadav, Nakulananda Mohanty, Satyabrata Dash, Shubham Pradhan, Bijayananda Sahoo and Biswajit Rath.
Data Availability Statement
All data and materials are available with corresponding authors.
Ethics Approval Statement:
Not Applicable.
References
- Nair R.M., Schafleitner R., Lee, S.H. The Mungbean Genome: Springer 679 International Publishing (Compendium of Plant Genomes). 2020. doi: https://doi.org/10.1007/978- 680 3-030-20008-4.
CrossRef - Hall C., Hillen C., Garden Robinson J. Composition, nutritional value, and health benefits of pulses. Cereal Chem.2017;94:11–31. doi: 10.1094/CCHEM-03-16-0069-FI
CrossRef - Singh B., Singh J. P., Shevkani K., Singh N., Kaur A. Bioactive constituents in pulses and their health benefits. Food Sci. Technol.2017;54:858–870. doi: 10.1007/s13197-016-2391-9.
CrossRef - Mubarak A. E. Nutritional composition and antinutritional factors of Mungbean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem.2005;89:489–495. doi: 10.1016/j.foodchem.2004.01.007.
CrossRef - Yi-Shen Z., Shuai S., Fitz Gerald R. Mungbean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res.2018,62 doi: 10.29219/fnr.v62.1290.
CrossRef - Dahiya P. K., Linnemann A. R., Van Boekel M. A. J. S., Khetarpaul N., Grewal R. B., Nout M.J.R. Mungbean Technological and nutritional potential. Rev. Food Sci. Nutr.2015;55:670–688. doi: 10.1080/10408398.2012.671202.
CrossRef - Kabre J. d. A. W., Dah-Nouvlessounon D., Hama-Ba F., Agonkoun A., Guinin F., Sina H., Kohonou A. N., Tchogou P., Senou M., Savadogo A., Baba-Moussa L. Mungbean (Vigna radiata(L.) R. Wilczek) from Burkina Faso used as antidiabetic, antioxidant and antimicrobial agent. Plants. 2022;11: 3556.
CrossRef - Dianzhi H., Laraib Y., Yong X., Jinrong H., Jihong W. Mungbean (Vigna radiata): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits Nutrients. 2019; 11: 1238.
CrossRef - Zafar, S. H., Umair M., Akhtar M. Nutritional evaluation, proximate and chemical composition of Mungbean varieties/cultivars pertaining to food quality characterization. Food Chem. Adv.2023; 2: 100160.
CrossRef - Singh, R. P. Comparative analysis: Area, production and yield of major pulse crops growing states in India. Status paper on pulses. 2010; pp.3-8.
- Madhujith T., Naczk M., Shahidi F. Antioxidant activity of common beans (Phaseolus vulgaris L.). J Food Lipids. 2004; 11:220-233.
CrossRef - Ahmad M. S. A., Hussain M., Ijaz S., Alvi A. K. Photosynthetic performance of two Mungbean (Vigna radiata (L.) cultivars under lead and copper stress. Int J Agri Biol. 2008;10:167-172.
- Lambrides C. J., Godwin, I. D. In: Kole, C., Ed., Genome Mapping and Molecular Breeding in Plants—Pulses, Sugar and Tuber Crops. 2007; Springer, Heidelberg, Germany, 69-90.
CrossRef - Miah M. A., Anwar K., Begum M. P., Juraimi M. A. S. Islam, M. A. Influence of sowing date on growth and yield of summer Mungbean varieties. J. Agric. Soc. Sci. 2009; 5: 73-76.
- Kumar S., Chauhan P. S., Agrawal L., Raj R., Srivastava A., Gupta S. Paenibacillus lentimorbus inoculation enhances tobacco growth and extenuates the virulence of cucumber mosaic virus. PLoS One.2016;11:e0149980.doi:10.1371/journal.pone. 0149980
CrossRef - Teaumroong N., Wanapu C., Chankum Y., Arjharn W., Sang-Arthit S., Teaimthaisong K., Boonkerd N. Production and application of bioorganic fertilizers for organic farming systems in Thailand: a case study. In: Microbes at work. Berlin: Springer. 2010; 293–312. Doi:10.1007/978-3-642-04043-6_15.
CrossRef - Watanabe F. S., Olsen S. R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil1. Soil Science Society of America Journal, 1965; 29(6): 677. doi:10.2136/sssaj1965.03615995002900060025x
CrossRef - Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Molecular biology and evolution, 2021; 38(7): 3022-3027. https://doi.org/10.1093/molbev/msab120
CrossRef - Ahemad, M., Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King saud University-science, 2014; 26(1): 1-20.
CrossRef - Yadav A. N., Sachan S. G., Verma P., Kaushik R., Saxena A. K. Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. J Basic Microbiol. 2016; 56:294–307
CrossRef - Yadav A. N., Verma P., Kaushik R., Dhaliwal H. S., Saxena A. K. Archaea endowed with plant growth promoting attributes. EC Microbiol. 2017a; 8:294–298
- Yadav A.N., Verma P., Kour D., Rana K.L., Kumar V., Singh B., Chauahan V.S., Sugitha T., Saxena A.K., Dhaliwal H.S. Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int J Environ Sci Nat Resour. 2017b; 3:1–8
CrossRef - Verma P., Yadav A.N., Kazy S.K., Saxena A.K., Suman A. Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int J Curr Microbiol Appl Sci. 2014; 3:432–447
- Verma P., Yadav A. N., Khannam K. S., Panjiar N., Kumar S., Saxena A. K., Suman A. Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol. 2015b; 65:1885–1899
CrossRef - Verma P., Yadav A. N., Khannam K. S., Kumar S., Saxena A. K., Suman A. Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol. 2016a; 56:44–58.
CrossRef - Suman A., Yadav A. N., Verma P. Endophytic microbes in crops: diversity and beneficial impact for sustainable agriculture. Microbial inoculants in sustainable agricultural productivity. Research perspectives. 2016; 1: 117-143.
CrossRef - Pandey P., Maheshwari D. K. Two-species microbial consortium for growth promotion of Cajanus cajan. Current science, 2007; 1137-1142.
- De Souza R., Beneduzi A., Ambrosini A., Da Costa P. B., Meyer J., Vargas L. K., Passaglia L. M. The effect of plant growth-promoting rhizobacteria on the growth of rice (Oryza sativa) cropped in southern Brazilian fields. Plant and soil, 2013; 366: 585-603.
CrossRef - Yadav C., Pandey S. Isolation and characterization of phosphate solubilizing bacteria from agriculture soil of Jaipur, Rajasthan. International Journal of Current Trends in Science and Technology. 2018; 8(01).
- Qureshi M. A., Shakir M. A., Iqbal A., Akhtar N., Khan A. Co-inoculation of phosphate solubilizing bacteria and rhizobia for improving growth and yield of Mungbean (Vigna radiata). J. Anim. Plant Sci, 2011; 21(3):491-497
- Zaidi A., Khan M. S., Aamil M. Bio-associative effect of rhizospheric microorganisms on growth, yield and nutrient uptake of greengram. J Plant Nutr. 2004; 27:599–610.
CrossRef - Navsare R. I., Mane S. S., Supekar S. J. Effect of potassium and zinc solubilizing microorganism on growth, yield and quality of Mungbean. International Journal of Chemical Studies, 2018; 6(1), 1996-2000.
- Shivashankar K., Singh A., Singh S. Response of Summer Mungbean [Vigna radiata (L.) wilczek] to foliar potash fertilization under different moisture regimes. Indian Journal of Agricultural Research, 2023; 57(3): 342-346.
- Imran Asad Ali Khan., Inamullah Inam., Fayaz Ahmad. Yield and yield attributes of Mungbean (Vigna radiata ) cultivars as affected by phosphorous levels under different tillage systems, Cogent Food & Agriculture. 2016; 2:1, 1151982, DOI: 10.1080/23311932.2016.1151982.
CrossRef - Hussain I., Abbas G., Hussain J., Abbas Z., Mehmood T., Amer M., Maqsood Q., Rasool I. Application of sulphate of potash enhances Mungbean (Vigna radiata) yield under agroclimatic conditions of Thal, Pakistan. J. Environ. Agric. Sci. 2022; 24(3&4):23-28.

This work is licensed under a Creative Commons Attribution 4.0 International License.





