How to Cite | Publication History | PlumX Article Matrix
Deboja Sharma1, Satyakam Agarwala1*, Pranab Jyoti Koch1, Binapani Sanjrambam1 and Rajesh Singla2
1Department of Applied Biology, University of Science and Technology Meghalaya, Ri- Bhoi, India.
2Department of Microbiology, Saraswati Group of Colleges, Maur Mandi, Bathinda, Punjab India.
Corresponding Author E-mail: satya.agar2020@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3218
ABSTRACT: Biosurfactant producing microorganisms have a potential in mitigating environmental pollution contaminated with hydrocarbon. This study aimed to isolate biosurfactant-producing organisms from the soil contaminated with oil. Out of the eight isolates, six (P1, P2, P3, D1, X, and Y) produced biosurfactant, as confirmed by the oil displacement method, with isolate P3 showing 4.2 cm oil displacement activity. The emulsification assay revealed that samples P3 and X had the highest emulsification activity at 25%. The identification of biosurfactant was further confirmed by the foaming activity method and biosurfactant extraction with chloroform and methanol. The biosurfactant nature was determined by the presence of glycolipid or neutral lipid, with the Rf values calculated for each isolate, and P3 showing a value of 0.92. Besides biosurfactant production, samples X, Y, and D1 also exhibited biocontrol activity against Aspergillus niger and Aspergillus fumigatus, as confirmed by fungal growth inhibition percentage and electron micrographic studies. This suggests the potential application of these isolates in bioremediation and biocontrol of plant pathogens. Further identification can be confirmed through 16s rRNA sequencing.
KEYWORDS: Antagonistic activity; Biosurfactants; Emulsification; Oil spreading technique; Phytopathogenic fungi; SEM
Download this article as:| Copy the following to cite this article: Sharma D, Agarwala S, Koch P. J, Sanjrambam B, Singla R. In vitro Studies on Antagonistic Behaviour of Biosurfactant Producing Microorganism against Pathogenic Fungi Aspergillus niger and Aspergillus fumigatus. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Sharma D, Agarwala S, Koch P. J, Sanjrambam B, Singla R. In vitro Studies on Antagonistic Behaviour of Biosurfactant Producing Microorganism against Pathogenic Fungi Aspergillus niger and Aspergillus fumigatus. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/48LTwfj |
Introduction
Amphipathic compounds, referred to as surfactants, contain both polar heads and non-polar tails, which are hydrophilic and hydrophobic in nature, respectively 1. They accumulate at the interface within various liquid stages, particularly when there is a liquid/oil collision. When the concentration reaches precise phases, these molecules form micelles; this concentration is termed the crucial micelle concentration 2. Micelles are molecules that reduce their exterior tension, a feature of liquids that enables them to endure forces from outside within various phases of the liquid. As a consequence of this, oil gets eliminated from both soil and water using a surfactant’s properties. Surfactant compounds are often produced via synthetic or biological means 3. Excessive use of chemical-based surfactants has a negative impact on the environment 4. Biosurfactants are secondary metabolites generated by a wide variety of microbial organisms, predominantly fungi, bacteria, and yeast 5–7. These metabolites usually circulate externally or are embedded in cell surfaces. They act as antibacterial substances in microbiological warfare as well as serve a role in the sense of quorum. Plant disease limits the quantity and efficacy of fib re and food, including biofuel-producing plants, while agricultural activities seek to provide nourishment for the globe’s frantically rising population. The farmers invest hundreds of millions of dollars regulating diseases consistently without enough assistance from experts, which has detrimental effects such as ineffective disease control and contamination 8. Furthermore, ailments of plants may completely annihilate ecosystems in nature, raising ecological problems driven by habitat loss and bad land management 9. When locales rely on imported products to compensate for disease catastrophes, a nutritious diet often gets substituted by chemically processed foods, which may deteriorate current medical conditions. Numerous forms of fungi are believed to trigger serious illnesses in plants, resulting in a substantial decline in crop production. The fungal infections and diseases of crops are typically by far the most adverse pathogenic microbes, resulting in substantial harm to farming annually 10,11. Interestingly, microbial biosurfactants have recently sparked vital business attention because they deliver a number of benefits over synthetic alternatives, which make them appropriate for pharmaceutical use, food manufacturing, and agriculture 12,13. A series of studies have addressed a wide range of biological surfactant manufacturers derived from water-based locations and heavily contaminated or uncontaminated soil holding petroleum-based products 14,15. Numerous rhizosphere and crop-associated microorganisms developed biosurfactants, which indicate possible involvement in interactions between plants and microbes or in general agricultural practices 13,16. Relevant traits for biological surveillance are being extremely accurate against pathogenic organisms in plants, being inexpensive, having minimal negative environmental impact, and having insignificant waste disposal problems. Despite the precise mechanisms through which antagonist microorganisms impact populations of pathogenic microbes, they are not always prominent 10,17. In the present study, bacterial strains were screened for their antagonistic potential against some common and destructive fungi, such as Aspergillus niger and Aspergillus fumigatus. These pathogenic fungi are responsible for considerable economic loss due to their destructive effect on the environment. The bacterial strains were checked for antagonistic effects by the plate assay method followed by SEM to confirm the deleterious effects of bacterial strains on fungal pellets.
Materials and methods
Sample Collections
Bacterial colonies were isolated from oil-contaminated soil collected from Vishwakarma Garage near Lakhi Mandir Beltola, Guwahati-28, Assam. The sample was taken in a sterile falcon tube, transported to a laboratory, and was stored at 4°C for further analysis.
Isolation of Bacterial Colonies
An amount of 5 grams of soil sample and 100 ml of “Mineral Salt Medium” was prepared in different conical flasks, and 1 mL of diesel and petrol were added respectively to serve as carbon sources. To derive bacteria yielding biosurfactant, the mixture was agitated simultaneously at 200 rpm for a duration of 72 hours at 30°C in order to develop a well-dispersed suspension. Isolation was performed using the serial dilution method onto Salt Nutrient Agar. The plates were then incubated for 24–48 hours at 37°C. Bacterial colonies were studied based on morphological characteristics and were chosen for further analysis. The pure isolates were maintained on nutrient agar slants and were stored at 4°C for further studies.
Extraction and production of Biosurfactant
Colonies were inoculated in mineral broth containing 2% vegetable oil (olive oil), petrol, diesel, and kerosene and were incubated in an optimized condition for 24-48 hours in a shaker operating at 120 rpm, followed by centrifugation at 10,000 rpm for 20 mins at 4°C, and the supernatant was taken as a crude source. The pH of the supernatant was adjusted to 2.0 using 6N HCl and was left undisturbed at 4°C overnight. An equal volume of chloroform and methanol in a ratio of 2:1 was mixed with the supernatant and was agitated for complete mixing. The mixture was left undisturbed until it evaporated.
Measurement of surface tension
The surface tension of the cell-free broth was measured using the tensiometer (Wilhelm plate) method. For comparison with distilled water, a cleaned Wilhelm plate was used, and onto it, 50 ml of crude biosurfactant compounds were added. The relative tension of the surface values was then measured using the tensiometer and recorded after a period of 72 hours 18.
Thin Layer Chromatography
A silica gel plate was used, and a crude biosurfactant was added to it. The biosurfactant was then separated using a solvent-based technique involving chloroform: methanol: acetic acid: water (25:15:4:2) with different color developing reagents. The glycolipid biosurfactant molecules were identified as having a yellow coloration using the Anthrone reagent, and the lipopeptides in the biosurfactant were confirmed to have a red discoloration using the ninhydrin reagent.
Oil spreading Technique
The evaluation of surfactant oil dispersing ability was carried out in the dispersion of oils 19. In a petri plate, 50 ml of deionized water and 10 µl of crude oil were mixed, resulting in the formation of a thin outer layer of oil. Subsequently, 10 µl of culture filtrate was precisely poured at the center of the crude oil coating. If surfactant molecules were found in the supernatant, they replaced the crude oil, and the area turned clear. The circumference of the clearance area was referred to as oil expulsion activity.
Emulsification Assay
Emulsifying activity was determined using E24, known as an emulsification index method with little modification as described by 20. For the emulsifying activity test, 2 ml of crude oil was added to a test tube. After centrifugation, the supernatant was vortexed for five minutes to ensure consistent mixing of the liquids. Within 24 hours, the emulsifying rate was recorded, and the activity was determined using the following specific formula:
E24 is calculated as the percentage of the thickness of the emulsifier phase divided by the entire height of the liquid column 21.
Foaming activity
Foaming activity was determined following the method given by Abou Seoud et al., 22. Bacterial isolates were maintained in nutrient broth medium for a period of 72 hours, with continuous agitation at 200 rpm at 37°C. Foamy action is determined by the period of its stability, foamy elevation, or foamy shape in the graduated cylinder.
Fungal Strains
Fungal strains of Aspergillus niger and Aspergillus fumigatus were collected from the laboratory of the Department of Microbiology, College of Veterinary Science, Khanapara bearing ATCC 16888 and ATCC 204305. The isolates were maintained on Czapek dox agar and potato dextrose agar under optimum conditions.
In vitro siderophore assay
The chrome-azurol S (CAS) was used to determine if siderophores were generated by the isolated cells 23. Blue agar was prepared by homogeneously mixing 60.5 mg of chrome-azurol S in 50 ml of double-distilled water with a 10 ml Fe (III) solution. Subsequently, 40 ml of water containing 72.9 mg of hexadecyltrimethylammonium (HDTMA) was gradually mixed into the solution, resulting in a dark-colored solution, which was then autoclaved. A mixture containing 750 ml of distilled water, 15 g of agar, 30.24g of PIPE buffer (1000 mL distilled water 302.37g of Pipe), and 12g of 50% NaOH solution (pH 6.8) was autoclaved separately. After cooling the sterilized solution to 50°C, 30 ml of 10% casamino acid v/v was added. The colored solution was then added along the glass walls with adequate agitation to mix the components without producing foam. The media was then plated. By adding a 10 mM Fe (III) solution, the agar was given a bright blue color. Siderophores, excreted by iron-deficient microorganisms, generally exceeded this limit, leading to a shift in the entire color shade to orange.
In vitro antagonistic activity
Yeast Extract Mannitol (YEM) agar media was used to culture both bacterial and fungal isolates. The capacity of the isolated bacteria to suppress the growth of fungal strains on yeast extract mannitol agar plates was then assessed. The petri plates were subsequently kept at 27 °C for 5-7 days and observed for clear zone of inhibition indicating cessation of fungal growth.
Scanning Electron Microscopy
The fungal isolates growing towards the zone of interaction with the bacterial isolate were subjected to scanning electron microscopy (SEM). The sample was air-dried, and an agar disc of 1mm thickness was cut from the center of the petri plates, serving as a control sample. Another sample was prepared by cutting an agar disc of 1mm thickness from the edges of the point of interaction and placing it on cover glasses. These samples were coated with gold or palladium (Au or Pd coating) and mounted on an aluminum stub before imaging and scanning using FE-SEM Zeiss Sigma VP with HT voltage.
Results and Discussions
Isolation of Bacteria and screening of biosurfactant activity
Biosurfactants are useful in hydrocarbon bioremediation because they support the growth of microorganisms and consume hydrocarbon contaminants. Microorganisms can produce a wide variety of biosurfactants, which are environmentally friendly as compared to chemical surfactants 24. In the presence study biosurfactant microorganism were isolated from oil spilled soil from a local garage situated in Lakhi Mandir area of Beltola, Guwahati-28, Assam. Eight bacterial isolates (P1, P2, P3, D1, D2, D3, Y, and X) with different morphologies were isolated and cultured on nutrient agar media, and stored at 4°C for further study.
Surface tension measurement
The oil spreading and surface tension assays were found to be directly correlated. There was a marked reduction in the surface tension of cell-free culture broth. The results showed that the isolate “P3” had the highest reduction in surface tension compared to other samples, as indicated in Table 2. Surface tension reduction was also measured for other isolates, which indicated the production of surface-active compounds 25. Isolate Y showed a surface tension of 40.8 mN/m, and isolate X and D1 showed activity of 41.8 and 46.5 mN/m, respectively.
Thin layer chromatography
Thin-layer chromatography (TLC) showed a brown spot when the plate was sprayed with anthrone reagent, confirming the presence of amino acids. The brown spot indicated the presence of glycolipid-type biosurfactant. Rf value of 0.78 cm for P2, 0.88 cm for P3, 0.87 cm for D1, 0.88 cm for X, and 0.85 cm for Y were recorded. Rf value of 0.88 was consistent with mono-rhamnolipid moieties A Rf value of 0.6 cm was found in work carried out by 26. The results Rf values of isolates P1, P2, P3, D1, X, and Y, as shown in Table 3.
Oil spreading technique
Oil spreading assay showed positive results for the isolates. 27 explained that the oil displacement area is directly proportional to the surface-active compound in the solution. However, in this study, only the qualitative study was done to check the presence of surfactant. All the six bacterial isolates (P1, P2, P3, D1, X, and Y) were able to displace oil and clear zone was observed 28. The clear zone diameter was measured and the results are presented in Table 1. Oil displacement for isolates P1, P2, P3, D1, X and Y was compared to earlier work done by 29.
 |
Figure 1: a) Electronmicrograph showing control site of A.niger b) Image showing inhibition of of A.niger by isolate D c) Electronmicrograph showing control site of A.fumigatus |
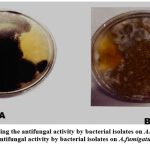 |
Figure 2: a) Showing the antifungal activity by bacterial isolates on A.niger b) Showing antifungal activity by bacterial isolates on A.fumigatus. |
Table 1: The oil spread technique’s result
| Sl. no | Bacterial
strain’s |
Clear zone diameter
(cm) |
Interpretation |
| 1 | P1 | 3.4 | +++ |
| 2 | P2 | 4.0 | +++ |
| 3 | P3 | 4.2 | +++ |
| 4 | D1 | 3.5 | +++ |
| 5 | X | 4.1 | +++ |
Table 2: Measurement of surface tension
| Sl.no | Sample | Time | Reading (mN/m) |
| 1 | P1 | 72 hrs | 46 |
| 2 | P2 | 72 hrs | 35.4 |
| 3 | P3 | 72 hrs | 50.9 |
| 4 | D1 | 72 hrs | 46.5 |
| 5 | X | 72 hrs | 41.8 |
| 6 | Y | 72 hrs | 40.8 |
Table 3: Result of Thin layer chromatography
| Sl.no | Bacterial
sample |
Distance moved by the
Molecule |
Distance moved by
the mobile phase |
Rf value |
| 1 | P1 | 13.2 | 17 | 0.78 |
| 2 | P2 | 15 | 17 | 0.88 |
| 3 | P3 | 15.6 | 17 | 0.92 |
| 4 | D1 | 14.9 | 17 | 0.87 |
| 5 | X | 15 | 17 | 0.88 |
| 6 | Y | 14.6 | 17 | 0.86 |
Emulsification Assay
The emulsification assay is a method used to screen for the production of biosurfactants. It is based on the assumption that if a cell-free culture broth contains biosurfactants, it will be able to emulsify hydrocarbons. In this study, crude oil was used as the hydrophobic substrate. The emulsification index (E24) of all six isolates is shown figure 3. The graph illustrates the emulsification activity of the bacterial isolates, with P3 and X having the highest E24% values compared to other isolates such as P1, P2, D1, and Y. The study investigated the potential of a strain of bacteria in emulsifying crude oil, and results showed that samples “X” and “P3” exhibited the highest emulsification activity at 25% in diesel as compared to samples Y, D1, P1, and P2, where other studies mention an emulsification assay of 70% 26. Emulsification is an indication that biosurfactants produced by the isolates can be used for enhancing bioremediation of hydrophobic environmental contaminants. The values are the average of three readings, and emulsification indices greater than 30% are indicated in bold to show high activity.
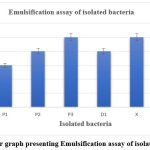 |
Figure 3: Bar graph presenting Emulsification assay of isolated bacteria. |
Foaming Activity
The foaming activity method confirmed that all six isolates (P1, P2, P3, D1, X, and Y) are biosurfactant producers. Foamy activity of all the isolates showed stability, foamy elevation in the graduated cylinder. The foaming activity of biosurfactant-producing microorganisms is a measure of their ability to produce surfactants that reduce surface tension and stabilize air-water interface, indicating biosurfactant production. This parameter can be used to screen for new biosurfactant-producing microorganisms and to optimize biosurfactant production conditions.
Siderophore Assay
In this study, the isolates X, Y, and D showed production of siderophore on CAS agar media. Several studies have reported the production of siderophores by different rhizobial strains 30,31. Several strains of Pseudomonas florescent have also been reported to produce siderophores 32–34. Siderophores are important metal-chelating agents, which are known to increase bioavailability of Fe to bacteria under Fe limiting conditions 35. In oil-rich environments, microbes associated with oil production formed amphiphilic siderophores, which are highly advantageous. These microorganisms, including oil-degrading bacteria, produce biosurfactants to disperse and emulsify hydrocarbons Amphibactins, due to their amphiphilic nature, serve as both siderophores and biosurfactants. This dual role serves in the process of iron uptake and hydrocarbon degradation 36 37. They prevent siderophore diffusion and enhance oil solubility at the bacterial membrane, facilitating efficient degradation of hydrocarbons.
Antagonistic activity and SEM
Isolates X, Y, and D showed biocontrol activity against Aspergillus niger and Aspergillus fumigatus. Similar studies were also carried out with Sinorhizobium meliloti against phytopathogenic fungi 38. The fungal growth inhibition percentage increased with an increase in the incubation period, this may be because of the release of secondary metabolites by the bacterial isolate D, X and Y along with incubation period 39.Isolate D inhibited the growth of Aspergillus fumigatus and Aspergillus niger by 70% after 5 days of incubation, whereas isolates X and Y were found to be effective by 60% after 5 days of incubation as shown in figure 4. Electron micrographic studies of isolates X, Y, and D showed clear morphological abnormalities including breakage and swelling in some parts of the hyphae and mycelium of Aspergillus niger and Aspergillus fumigatus confirming the antagonistic behavior of the isolates. It is observed that deformities in fungal hyphae of M. phaseolina caused by a strain of Pseudomonas florescent through the production of secondary metabolites40.
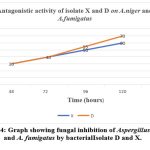 |
Figure 4: Graph showing fungal inhibition of Aspergillus niger and A. fumigatus by bacterial Isolate D and X. |
Summary
In this study, isolation and characterization of biosurfactant-producing organisms from oil- spilled soil provide valuable insights into their potential applications in various fields. The results of this study demonstrated the effectiveness of six isolates (P1, P2, P3, D1, X, and Y) in producing biosurfactants, which were confirmed through various assays, which includes oil displacement, emulsification, foaming activity, surface tension reduction, and lipid analysis. Moreover, the isolates showed biocontrol activity against pathogenic fungi and the ability to produce siderophores, highlighting their potential in bioremediation and biological control.
The antagonistic behaviour of the isolates against Aspergillus niger and A. fumigatus was confirmed through electron micrographic studies, which suggest that biosurfactants produced by these isolates have a potential role in controlling fungal infections. However, further studies are needed to identify the specific compounds responsible for this activity.
Conclusion
Biosurfactant production by microorganisms has variety of potential applications in various fields, including bioremediation and biological control. The underlying research includes the applications of microbes isolated from oil spills that can be used not only for minimizing hydrocarbon contamination but also for their antagonistic effects on pathogenic fungi. Various tests confirmed the isolates as biosurfactant producing microbes as well as how they can be effective against fungi, thereby, controlling fungal infections. However, additional studies are required to investigate the molecular mechanisms underlying the observed activities and to optimize the production of biosurfactants for its use in mitigation of environmental pollution.
Acknowledgement
The authors declare that no funds, grants, were received during the preparation of this manuscript. Authors are thankful to the Department of Applied Biology, University of Science and Technology Meghalaya, India for providing research facilities.
Conflict of Interest
Authors have no competing or conflict of interests.
Funding Sources
No funding received in making this manuscript
Authors’ Contribution
Authors are required to provide a statement detailing the specific contributions of each author to the manuscript.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study. If your manuscript does not contain any data, please state ‘Not applicable’ in this section.
Ethics Approval Statement
If the study involves an experiment on humans and animals; then the name of the authorizing body should be stated in the paper
References
- Ghosh S, Ray A, Pramanik N. Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophys Chem. 2020;265:106429.
CrossRef - Desai JD, Banat IM. Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews. 1997;61:47-64. https://api.semanticscholar.org/CorpusID:41445216
CrossRef - Moldes AB, Rodríguez-López L, Rincón-Fontán M, López-Prieto A, Vecino X, Cruz JM. Synthetic and bio-derived surfactants versus microbial biosurfactants in the cosmetic industry: An overview. Int J Mol Sci. 2021;22(5):2371.
CrossRef - Singh P, Patil Y, Rale V. Biosurfactant production: emerging trends and promising strategies. J Appl Microbiol. 2019;126(1):2-13.
CrossRef - Piegza M, Pietrzykowska J, Trojan-Piegza J, Łaba W. Biosurfactants from Trichoderma Filamentous Fungi—A Preliminary Study. Biomolecules. 2021;11(4):519.
CrossRef - Sansinenea E, Ortiz A. Secondary metabolites of soil Bacillus spp. Biotechnol Lett. 2011;33:1523-1538.
CrossRef - Ndlovu T, Rautenbach M, Vosloo JA, Khan S, Khan W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express. 2017;7(1):1-19.
CrossRef - Flood J. The importance of plant health to food security. Food Secur. 2010;2(3):215-231.
CrossRef - Harte J. Land use, biodiversity, and ecosystem integrity: the challenge of preserving earth’s life support system. Ecol Law Q. Published online 2001:929-965.
- Heydari A, Pessarakli M. A review on biological control of fungal plant pathogens using microbial antagonists. Journal of biological sciences. 2010;10(4):273-290.
CrossRef - Agrios GN. Plant Pathology. Elsevier; 2005.
- Nitschke M, Costa S. Biosurfactants in food industry. Trends Food Sci Technol. 2007;18(5):252-259.
CrossRef - Sachdev DP, Cameotra SS. Biosurfactants in agriculture. Appl Microbiol Biotechnol. 2013;97:1005-1016.
CrossRef - Rani M, Weadge JT, Jabaji S. Isolation and characterization of biosurfactant-producing bacteria from oil well batteries with antimicrobial activities against food-borne and plant pathogens. Front Microbiol. 2020;11:64.
CrossRef - Neethu CS, Saravanakumar C, Purvaja R, Robin RS, Ramesh R. Oil-spill triggered shift in indigenous microbial structure and functional dynamics in different marine environmental matrices. Sci Rep. 2019;9(1):1354.
CrossRef - Marchut-Mikolajczyk O, Drożdżyński P, Pietrzyk D, Antczak T. Biosurfactant production and hydrocarbon degradation activity of endophytic bacteria isolated from Chelidonium majus L. Microb Cell Fact. 2018;17(1):1-9.
CrossRef - Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52(suppl_1):487-511.
CrossRef - Abu‐Ruwaida AS, Banat IM, Haditirto S, Salem A, Kadri M. Isolation of biosurfactant‐producing bacteria, product characterization, and evaluation. Acta Biotechnol. 1991;11(4):315-324.
CrossRef - Morikawa M, Daido H, Takao T, Murata S, Shimonishi Y, Imanaka T. A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J Bacteriol. 1993;175(20):6459-6466.
CrossRef - Rahman KSM, Rahman TJ, Lakshmanaperumalsamy P, Marchant R, Banat IM. The potential of bacterial isolates for emulsification with a range of hydrocarbons. Acta Biotechnol. 2003;23(4):335-345.
CrossRef - Lai CC, Huang YC, Wei YH, Chang JS. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater. 2009;167(1-3):609-614.
CrossRef - Mouafi FE, Elsoud MMA, Moharam ME. Optimization of biosurfactant production by Bacillus brevis using response surface methodology. Biotechnology Reports. 2016;9:31-37.
CrossRef - Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160(1):47-56.
CrossRef - Chandran P, Das N. Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. Int J Eng Sci Technol. 2010;2(12):6942-6953.
- Banat IM, Samarah N, Murad M, Horne R, Banerjee S. Biosurfactant production and use in oil tank clean-up. World J Microbiol Biotechnol. 1991;7:80-88.
CrossRef - Tabatabaee A, Assadi MM, Noohi AA, Sajadian VA. Isolation of biosurfactant producing bacteria from oil reservoirs. J Environ Health Sci Eng. 2005;2(1):6-12.
- Mohsen LY, Jarallah EM, Al-mamoori AMJ. Screening And Characterization Of Biosurfactant-Producing Bacteria Isolated From Oil-Contaminated Soil. J Pharm Negat Results. Published online 2022:1325-1331.
- Urum K, Pekdemir T. Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere. 2004;57(9):1139-1150.
CrossRef - Chioma O, Ogechukwu M, Bright O, Simon O, Chinyere AF. Isolation and characterization of biosurfactants producing bacteria from oil polluted soil. Journal of Natural Sciences Research. 2013;3(5):119-123.
- Dudeja SS, Suneja S, Khurana AL. Iron acquisition system and its role in legume-Rhizobium symbiosis. Indian J Microbiol. 1997;37:1-12.
- Jaiswal SK, Mohammed M, Ibny FYI, Dakora FD. Rhizobia as a source of plant growth-promoting molecules: Potential applications and possible operational mechanisms. Front Sustain Food Syst. 2021;4:619676.
CrossRef - Martínez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr. 2010;10(3):293-319.
CrossRef - Gamalero E, Fracchia L, Cavaletto M, et al. Characterization of functional traits of two fluorescent pseudomonads isolated from basidiomes of ectomycorrhizal fungi. Soil Biol Biochem. 2003;35(1):55-65.
CrossRef - Marschner P, Crowley D, Rengel Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol Biochem. 2011;43(5):883-894.
CrossRef - Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160(1):47-56.
CrossRef - Bento FM, de Oliveira Camargo FA, Okeke BC, Frankenberger Jr WT. Diversity of biosurfactant producing microorganisms isolated from soils contaminated with diesel oil. Microbiol Res. 2005;160(3):249-255.
CrossRef - Yakimov MM, Golyshin PN, Lang S, et al. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int J Syst Evol Microbiol. 1998;48(2):339-348.
CrossRef - Deshwal VK, Singh SB, Kumar P, Chubey A. Rhizobia unique plant growth promoting rhizobacteria: a review. Int J Life Sci. 2013;2:74-86.
- Arfaoui A, Sifi B, Boudabous A, Hadrami I El, Cherif M. Identification of Rhizobium isolates possessing antagonistic activity against Fusarium oxysporum f. sp. ciceris, the causal agent of Fusarium wilt of chickpea. Journal of Plant Pathology. Published online 2006:67-75.
- Gupta CP, Dubey RC, Kang SC, Maheshwari DK. Antibiosis-mediated necrotrophic effect of Pseudomonas GRC 2 against two fungal plant pathogens. Curr Sci. Published online 2001:91-94.

This work is licensed under a Creative Commons Attribution 4.0 International License.






