How to Cite | Publication History | PlumX Article Matrix
R. Renugadevi* , M. P. Ayyappadas
, M. P. Ayyappadas , M. Mahesh
, M. Mahesh , M. Kiruba
, M. Kiruba and M. Arunkumar
and M. Arunkumar
Department of Biotechnology, RVS College of Arts and Science (Autonomous), Sulur, Coimbatore, Tamilnadu, India.
Corresponding Author E-mail: renugadevir2010@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3212
ABSTRACT: Nitrogen is essentially required for the plant growth as well as productivity. Plants take nitrogen in the form of ammonia or nitrate either from soil or fertilizer. There are nitrogen fixing bacteria plays a vital role to supply atmospheric nitrogen to plants where plants do not obtain from soil. Apart from soil bacteria, endophytic bacteria which living inside the plant tissues can improve crop productivity and soil health sustainably through biological nitrogen fixation and act as a potential replacement for chemical fertilizers in agriculture. This study was conducted to determine the nitrogen fixing activity of isolated endophytic bacteria from Kalanchoe pinnata (Lam.). The isolated endophytes were subjected to molecular confirmation and evaluated for ammonia production, Acetylene Reduction Assay (ARA), nif gene amplification and analysis of growth parameters in Zea mays using pot culture assay. The data were analyzed using SPSS ver.16. In this study, Bacillus thuringiensis, Bacillus paranthracis, Staphylococcus xylosus and Bacillus cereus were isolated from the leaves of Kalachoe pinnata (Lam). They were confirmed using 16SrRNA sequencing. All the endophytic bacteria were positive for ammonia production and ARA. The percentage of nitrogen produced was 32.8 % (B. thuringiensis), 65.7% (B. paranthracis), 80.7% (S. xylosus) and 45.2% (B. cereus).The presence of nif gene was confirmed through the PCR amplification of a 550-580bp fragment of the gene. Pot culture assay of Zea mays were observed with significant improvement in S. xylosus followed by B. paranthracis inoculated pots. The presence of the nitrogenase enzyme and the nif gene in these endophytic bacteria allows them to fix atmospheric nitrogen to meet plant nitrogen demands, resulting in a symbiotic relationship with agricultural crops.
KEYWORDS: Acetylene Reduction Assay; Ammonia Production; nif gene; Zea mays
Download this article as:| Copy the following to cite this article: Renugadevi R, Ayyappadas M. P, Mahesh M, Kiruba M, Arunkumar M. Nitrogen Fixing Activity of Endophytic Bacteria Associated with Kalanchoe pinnata (Lam.) and its effect on Zea mays. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Renugadevi R, Ayyappadas M. P, Mahesh M, Kiruba M, Arunkumar M. Nitrogen Fixing Activity of Endophytic Bacteria Associated with Kalanchoe pinnata (Lam.) and its effect on Zea mays. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/3wV73TR |
Introduction
Nitrogen fixation is the most important biological nitrogen cycle. The presence of nitrogen in molecules other than N2 is frequently the limiting factor for plant growth. Fixation takes place through industrial, atmospheric, and biological processes. In the atmosphere, nitrogen is converted into nitrogen compounds for biochemical processes.
Biological nitrogen fixation is seen to be the most promising method of supplying plants with fixed forms of nitrogen. It occurs through the conversion of N2 to NH3, NH4+, or organic nitrogen under the influence of an enzyme. In general, prokaryotes and archaea are the only organisms capable of fixing nitrogen but many research works stated that endophytic bacteria work in symbiosis with them and can uses the nitrogenase enzyme to fix atmospheric nitrogen into ammonia1,2. It was noted that ammonia production test, nifH gene amplification, and Acetylene Reduction Assay (ARA) help to confirm N fixing ability3.
In recent years, the application of endophytic bacterial inoculants supplying N has drawn attention for increasing plant yield in a sustainable manner in various crop plants. Besides, endophytes were claimed to increases the insect resistance, stress tolerance, synthesize enzymes or peptides providing nutrients, as well as replacing the chemical fertilizers because it producing the plant growth hormones such as indole acetic acid and cytokinin4,5. Nitrogen fixing endophytic bacteria was present in agricultural crops as well as medicinal plant parts such as roots, stems, fruits, and leaves6.7. Pseudomonas, Microbacterium, Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes are few endophytic bacteria identified for nitrogen fixing activity8.
Kalanchoe pinnata belongs to Crassulaceae, commonly known as miracle leaf and life of leaf distributed in tropical and subtropical regions of Asia. The elliptic, 20 cm tall leaf or leaflets have a notched border that generates plantlets9. This plant is a medicinal plant that has normally been used in ayurvedic medicines since ancient times. Despite their medicinal importance, many studies were not undertaken in evaluating the endophytes associated with this plant. In this regards, our study aims at i) Isolation of endophytic bacteria associated with leaves of Kalanchoe pinnata (Lam.) ii) Characterization of endophytic bacteria, iii) Determination of nitrogen fixing activity and iv) Study of growth promotion activity in Zea mays (figure 1). Overall, this research provides valuable potential information about nitrogen-fixing endophytic bacteria that could be used for producing bio-fertilizer which may be effective in agriculture.
 |
Figure 1: Nitrogen Fixing Activity of Endophytic Bacteria |
Materias and Methods
Collection, Identification and Sampling of Plant material
The plant samples were collected from farms in and around Coimbatore district and the plants were identified using the service of the botanical survey of India, TNAU, Coimbatore, Tamilnadu. Fresh leaves were collected in a Ziploc cover under aseptic condition and washed 3 times with running tap water to remove surface soils. Surface sterilisation was performed using 70% ethanol for 5 min and 2% sodium hypochlorite for 1 min, subsequently the samples were washed 3 times with sterile water10,11.
Isolation, Morphological and Biochemical Characteristics of Endophytic Bacteria
Fresh leaves were ground in 2% saline, serially diluted, and plated in LB agar for isolation of nitrogen fixing endophytic bacteria. After 24 h, morphologically diverse bacterial colonies were chosen, and streaking was repeated until pure culture, andthe same were stored at 4°C till further use12.The morphological characters and biochemical parameters were assessed13.
Molecular identification of endophytic bacteria
Genomic DNA was isolated from selected endophytic strains14 and the amplification of 16S rRNA sequencing were performed using 27F (AGAGTTTGATCCTGGCTCAG) and 1492R (CGGTTACCTTGTTACGACTT) primers to amplify approximately 1500 bp of 16S rDNA gene15. PCR results were viewed and purified using a kit from Gene Aid16. Then, the products were sequenced at Biokart India Pvt. Ltd., Bengaluru, India.
Ammonia Production Test
The isolates were tested using qualitative method for the ammonia production17. Fresh culture of Bacillus thuringiensis, Bacillus paranthracis, Staphylococcus xylosus and Bacillus cereus were inoculated in peptone water and incubated at 30°C for48 to 72 h. The formation of a brown to yellow colour on Nessler’s reagent indicates a positive result18.
Determination of Nitrogenase Activity
Nitrogenase activity of isolates was tested using acetylene reduction assay. The isolated endophytes were incubated at 30°C for 48 h in semisolid nitrogen free media sealed tubes. After 48 h of incubation, the tubes were added with 10% acetylene and the tube without acetylene serves as control. After 24 hours, 0.25 ml of gas sample was collected and used for assessing the amount of ethylene formed using Gas chromatography fitted with Poropak R column at the flow rate of 2.5ml/min using Flame Ionisation Detector19,20,21.
Nitrogen Estimation
The endophytic cultures were collected from nitrogen free media containing 0.05% malate as carbon source incubated at 30°C, centrifuged and supernatant was separated for the estimation of nitrogen using Kjeldal method22.
nifH Gene Amplification
The DNA isolated from endophytic bacteria cultured in nitrogen free media and was used for assessing amplification of nifH quality. PCR was performed using primer sets of nifHF (5′- GGCAAGGGCGG TATCGGCAAGTC-3′) and nifHR (5′- CCATCGTGATCG GGTCGGGATG-3′). The nifH quality amplification was done in a complete volume of 25 µL containing Ultrapure water, 5x Taq buffer , 50 µM of each primer, 25 mM MgCl2, 100 µM dNTPs, 5U/µL Taq polymerase, and DNA template. The PCR conditions was as follows as: initial denaturation at 95°C for 90 sfollowed by 30 cycles of denaturation at 94°C, for 60 s; annealing at 57°C, for 60 s; elongation at 72°C, for 60 s and final extension at 72°C for 5 min. PCR products were resolved on a 2% agarose gel. Gel was stained with ethidium bromide solution (5µg/mL) for 30 min, washed with 1X TAE support and the DNA bands were visualized under UV transilluminator23.
Effect of Plant Growth Studies
Seed preparation and Inoculumn coating on Zea mays for Plant Growth enhancing property
The seeds of Zea mays for the tests were purchased from a local farmer in Pappampatti, Coimbatore, and surface-sterilized using 75% ethanol (v/v) for 30 seconds and 0.2% of freshly prepared mercury chloride for three seconds and three washes with sterile distilled water was done to remove excess mercury chloride. 0.1ml of an overnight grown culture was applied as a seed coating, dried, and sown as a carrier into sterile soil. The experiment was carried out in triplicates for each isolate and twelve seeds were sowed in each pot at an equal distance. As a control, seeds without coating were used. Every 15 days, the length of shoots and roots were measured, fresh and dry weight was noted, flowering stage and corn formation were observed 24.
Results and Discussion
Collection, Identification and Sampling of Plant material
The plant sample was collected from farm in Sulur, Coimbatore, identified with botanical survey of India, TNAU, Coimbatore, Tamilnadu and it was identified as Kalanchoe pinnata (Lam.).
Isolation, Biochemical and Morphological Characteristics of endophytic bacteria
In total, twenty seven bacterial endophytes were isolated from the leaves of Kalanchoe pinnata (Lam). After the preliminary screening test, we have selected 4 isolates/strains named as EB1, EB2, EB3 and EB4 for further study based on biochemical and morphological characters (table 1 and table 2). In a similar study, Mokhichekhra et al., (2021) isolated 7 strains from different parts of Kalanchoe degremona including roots, stem, and leaves25.
Table 1: Molecular identification of strain with Gene Bank Accession No. and Morphological Characters of isolates
| S.
No |
Strain | Name of Isolates | Gen Bank Accession No | Morphological Characters of Isolates | ||||||
| Gram staining | Shape | Form | Surface | Colour | Margin | Elevation | ||||
| 1 | EB1 | Bacillus thuringiensis | OM349623 | Positive | Rod | Irregular | Rough | Cream | Lobate | Flat |
| 2 | EB2 | Bacillus paranthracis | OK135976 | Positive | Rod | Circular | Smooth | Cream | Entire | Raised |
| 3 | EB3 | Staphylococcus xylosus | OM350007 | Positive | Round | Smooth | Shiny | Orange | Undulate | Flat |
| 4 | EB4 | Bacillus cereus | OK135977 | Positive | Rod | Circular | Smooth | Cream | Entire | Raised |
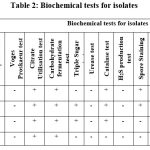 |
Table 2: Biochemical tests for isolates |
Molecular identification of endophytic bacteria
Among the four strains, EB1, EB2, and EB4 were categorized as Bacillus sp and EB3 as Staphylococcus sp based on their biochemical and morphological characteristics. For accurate identification, 16SrRNA sequence analysis was done. Clustal X was used for multiple-sequence alignment26 and sequences have deposited in the GenBank database which were designated under the following accession numbers: OM349623 (EB1- Bacillus thuringiensis), OK135976 (EB2-Bacillus paranthracis), OM350007 EB3-Staphylococcus xylosus) and OK135977 (EB4-Bacillus cereus) given in table 1. The family members of Firmicutes, Bacteroidetes, and Actinobacteria are generally reported to be the most frequent and predominant endophytes associated with plants27. Our results similarly revealed that Firmicutes (Bacillus sp.) are predominantly associated with this plant.
Ammonia Production Test
The production of ammonia is considered to be an indirect factor of growth promotion by endophytes in plants and also indicates the capability of endophytes to offer nitrogen to the plants28. The formation of orange to brown colour indicates a positive result for ammonia production. A study by Borah et al., (2019) for instance, observed the maximum ammonia production in Brevibacterium sp visibly with brown colour18. Here, B. thuringiensis, B. paranthracis, S. xylosus,and B. cereus were showed positive result to ammonia production (table 3 and figure 2). These isolated nitrogen fixing endophytes shown maximum ammonia production in 2nd and 3rd day compared to the 1st day culture filtrate. Similar results were also reported by Kang et al., (2020)29 that, highest level of ammonia was observed in the 2nd and 3rd day culture of Klebsiella pneumoniae than first day culture filtrate.
 |
Figure 2: Ammonia Production Test |
C: Control (Un inoculated Broth) shows Negative test, B. thuringiensis, B. paranthracis, S. xylosus and B. cereus shows orange colour indicating positive results
Table 3: Ammonia Production Test, Nitrogenase activity (ARA), and Percentage of Nitrogen
| S.No | Name of Isolates | Ammonia Production test | Nitrogenase Activity(ARA)
nmole/h/vial |
Percentage of Nitrogen (%) |
| 1 | B. thuringiensis | Positive | 82.6 | 32.77 ±0.07 |
| 2 | B. paranthracis | Positive | 167.2 | 65.73 ±0.06 |
| 3 | S. xylosus | Positive | 227.3 | 80.63 ±0.07 |
| 4 | B. cereus | Positive | 91.1 | 45.2 ±0 |
Determination of Nitrogenase Activity
All four endophytes, B. thuringiensis, B. paranthracis, S. xylosus,and B. cereus showed positive for nitrogenase activity; the results were expressed in nm/h/v (table 3 and figure 3). Similar to our results, previous research study also stated that, Pseudomonas sp, Bacillus sp, Klebsiella sp,and Paenibacillus sp shown maximum nitrogense activity using ARA1,2.. As well known the nitrogen fixation is generally catalysed by enzyme nitrogenase which mainly governs the ATP-dependent reduction of N2 (dinitrogen) to NH3 (ammonia) and are encoded by nif genes30. It was noted that, evaluation of N fixation was done frequently in free living bacteria as well as in endophytic bacteria. In addition, in situ acetylene reduction method was adopted to determine the nitrogenase activity31. Here, we followed acetylene reduction assay for nitrogenase activity, S. xylosus produced higher amount of ethylene (227.3nm/h/v) compared to B. paranthracis (167.2nm/h/v) and B. cereus (91.1nm/h/v) whereas B. thuringiensis produced loweramount of ethylene (82.6nm/h/v). Nitrogen fixation is an ecologically important process as an input for nitrogen into the habitat and equally representing the promising alternate for chemical nitrogen fertilizers32.
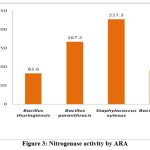 |
Figure 3: Nitrogenase activity by ARA |
Nitrogen Estimation
Kjeldahl method is commonly used for nitrogen estimation. Previous study stated that, Bacillus subtilis was prominent nitrogen fixing endophytes in saline condition and shown to contain highest nitrogen content in chickpea leaves33. It was noticed that Herbaspirillum frinsingense and Pantoea dispersa strains were actively participated in nitrogen fixing activity34. This study showed that B. thuringiensis produced 32.8% nitrogen, B. paranthracis produced65.7% S. xylosus produced 80.7% and B. cereus produced 45.2% of nitrogen based on Kjeldahl method. S. xylosus produced a higher percentage of nitrogen compared to B. thuringiensis, B. cereus,and B. paranthracis (table 3 and figure 4).
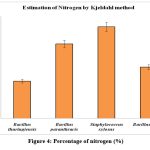 |
Figure 4: Percentage of nitrogen (%) |
nifH Gene Amplification
The nifH, an important part of nitrogenase protein complex II which is the most commonly used biomarkers for detecting the nitrogen fixing endophytes35. Genomic DNA (30ng) was used for PCR amplification fornif gene sequence. The primer was designed to amplify 600bp of nif gene fragment. Amplified nif gene was 550bp in B. thuringiensis, 580bp in B. paranthracis, 590bp in S. xylosus, and 580bp in B. cereus. All the active N2 fixers carry this nifHgenes encoding various nitrogenase complexes36. Our results confirmed the presence of nifH gene in all the isolated endophytes (figure 5); however the expression levels need to be evaluated.
 |
Figure 5: Characterization of nif genes of endophytes using nif H Primer |
Lane M: Marker, Lane 1: Bacillus thuringiensis, Lane 2: Bacillus paranthracis, Lane 3: Staphylococcus xylosus and Lane 4: Bacillus cereus
Effect of Plant Growth Studies
The effective study of endophytic inoculum on growth and nutrient performance were studied in various research fields. Chickpea37, rice seeds38, Zea mays39, groundnut and tomato40 were used for plant growth studies like the shoot and root length, weight of dry and fresh plants, number of fruits, flowers, and nutritional content. This study also focused on the effect of nitrogen fixation of endophytic bacteria to improve the growth of Zea mays. The length of root and shoot, weight of fresh and dry plants, flowering days, and corn appearance were noted. All four isolated endophytes showed positive significance in the promotion of maize growth. Previous similar study, YSD YN2 was treated with green grocery showed an increased level of chlorophyll content41.
The isolated endophytic isolates were treated on Zea mays seeds for growth promotion, growth effects were studied in every 15 days for 90 days. B. thuringiensis has increased the shoot length gradually from 26.25 ± 0.49 to 85.95 ± 0.09 cm, root length from 8.05 ± 0.09 to 25.15 ± 0.09 cm, fresh weight of the plant from 3 ± 0 to 15 ± 0, and dry weight from 0.85 ± 0.09 to 4.29 ± 0.01 gm, B. paranthracis were shown to increased the shoot length from 38.15 ± 0.29 to 112.1 ± 0 cm, root length from 10.1 ± 0 to 32 ± 0 cm, fresh and dry weight of the plants from 4.05 ± 0.10 to 33.15 ± 0.10 cm, and 1.20 ± 0.01 to 6.71 ± 0.02 gm, S. xylosus have increased the shoot and root length from 30.5 ± 0.98 to 104.05 ± 0.09 cm and 9.05 ±0.10 to 33.1 ±0 cm respectively, and fresh and dry weight from 3.15 ± 0.09 to 28.05 ± 0.09 gm and 1 ± 0 to 6.25 ± 0.09 gm, and B. cereus shown to increased the shoot and root length from 30.5 ± 0.98 to 97.1 ± 0.19 and 9 ± 0 to 28.05 ± 0.09 cm, fresh and dry weight of the plants was increased from 3.1 ± 0 to 23 ± 0 and 1 ± 0 to 5.31 ± 0.02 gm. It was observed that in all isolates, shoot, and root length significantly increased from 15 to 75 days, but only slight changes or no changes were noted after 75 to 90 days once flowers and corns appeared compared to the control (table 4, 5 and figure 6 & 7). Previous research reports stated that Mixta theicola strain was isolated from the roots Solenostemma argel, the root and seedlings, fresh and dry weight of Zea mays was shown to increase significantly compared to the control42. This study concluded that all four isolated endophytes were effectively involved in nitrogen fixation and influences the growth of Zea mays.
Table 4: Effect of endophytes on shoot and root length, fresh and dry weight of Zea mays
| Days | Name of the isolate | Shoot Length (cm) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) |
| 15 | Control | 16 ±0 | 3 ±0 | 2.1 ±0 | 0.3 ±0 |
| B. thuringiensis (EB1) | 26.25 ±0.49 | 8.05 ±0.09 | 3 ±0 | 0.85 ±0.09 | |
| B. Paranthracis (EB2) | 38.15 ±0.29 | 10.1 ±0 | 4.05 ±0.10 | 1.20 ±0.01 | |
| S. xylosus (EB3) | 30.5 ±0.98 | 9.05 ±0.10 | 3.15 ±0.09 | 1 ±0 | |
| B. cereus (EB4) | 30.5 ±0.98 | 9 ±0 | 3.1 ±0 | 1 ±0 | |
|
30 |
Control | 35 ±0 | 5.1 ±0 | 5.05 ±0.09 | 0.92 ±0.01 |
| B. thuringiensis (EB1) | 45.05 ±0.10 | 9.1 ±0 | 7.1 ±0 | 1.59 ±0.02 | |
| B.paranthracis (EB2) | 75 ±0 | 13.05 ±0.09 | 12.15 ±0.10 | 2.41 ±0.01 | |
| S. xylosus (EB3) | 65.05 ±0.10 | 12 ±0 | 11.1 ±0 | 2.05 ±0.09 | |
| B. cereus (EB4) | 63.9 ±0.19 | 10.15 ±0.09 | 11 ±0 | 2.1 ±0 | |
| 45 | Control | 44.15 ±0.10 | 8.05 ±0.10 | 7.1 ±0 | 1.49 ±0.01 |
| B. thuringiensis (EB1) | 52.15 ±0.09 | 14.15 ±0.09 | 9.1 ±0 | 2 ±0 | |
| B.paranthracis (EB2) | 83 ±0 | 21 ±0 | 17 ±0 | 2.65 ±0.09 | |
| S. xylosus (EB3) | 75.45 ±0.09 | 20.15 ±0.09 | 12.1 ±0 | 2.41 ±0.02 | |
| B. cereus (EB4) | 72.95 ±0.09 | 20 ±0 | 9.1 ±0 | 2.45 ±0.09 | |
| 60 | Control | 74.95 ±0.09 | 15.1 ±0 | 10.1 ±0 | 2.31 ±0.01 |
| B. thuringiensis (EB1) | 82 ±0 | 21.15 ±0.09 | 11 ±0 | 4.05 ±0.09 | |
| B.paranthracis (EB2) | 104.9 ±0.19 | 28.1 ±0 | 25.1 ±0 | 6.51 ±0.01 | |
| S. xylosus (EB3) | 97.95 ±0.09 | 28 ±0 | 16 ±0 | 5.85 ±0.09 | |
| B. cereus (EB4) | 94.75 ±0.49 | 23.05 ±0.13 | 14.15 ±0.09 | 5.6 ±0 | |
| 75 | Control | 75.15 ±0.09 | 20.05 ±0.09 | 15 ±0 | 2.53 ±0.06 |
| B. thuringiensis (EB1) | 86 ±0 | 25 ±0 | 15.1 ±0 | 4.32 ±0.01 | |
| B.paranthracis (EB2) | 112.05 ±0.09 | 32.1 ±0 | 30.05 ±0.09 | 6.7 ±0 | |
| S. xylosus (EB3) | 104 ±0 | 33 ±0 | 25.15 ±0.09 | 6.05 ±0.09 | |
| B. cereus (EB4) | 96.95 ±0.09 | 28.1 ±0 | 23 ±0 | 5.31 ±0.01 | |
| 90 | Control | 75 ±0 | 20.1 ±0 | 15.15 ±0.09 | 2.49 ±0.01 |
| B. thuringiensis (EB1) | 85.95 ±0.10 | 25.15 ±0.09 | 15 ±0 | 4.29 ±0.01 | |
| B.paranthracis (EB2) | 112.1 ±0 | 32 ±0 | 33.15 ±0.10 | 6.71 ±0.02 | |
| S. xylosus (EB3) | 104.05 ±0.09 | 33.1 ±0 | 28.05 ±0.09 | 6.25 ±0.09 | |
| B. cereus (EB4) | 97.1 ±0.19 | 28.05 ±0.09 | 23 ±0 | 5.31 ±0.02 |
Table 5: Effect of endophytes on flower and corn appearance of Zea mays
| S.No | Name of the isolate | Flower Appearance | Corn Appearance |
| 1 | Control | 75days | 77days |
| 2 | B. thuringiensis (EB1) | 70days | 72days |
| 3 | B.paranthracis (EB2) | 68days | 69days |
| 4 | S. xylosus (EB3) | 69days | 71days |
| 5 | B. cereus (EB4) | 70days | 72days |
 |
Figure 6: Effect of Endophytes on shoot and root length of Zea mays in a) 15 days, b) 30 days, c) 45 days, d) 60 days, e) 75 days and f) 90 days |
 |
Figure 7: Effect of Endophytes on fresh and dry weight of Zea mays in a) 15 days, b) 30 days, c) 45 days, d) 60 days, e) 75 days and f) 90 days |
Conclusion
Nitrogen fixation is considered as important biological process where nitrogen gas is transformed into ammonia and nitrogenous compounds that plants and other microorganisms can use. Endophytic microorganisms live asymptomatically inside their hosts and aid plant growth, development, fitness, and diversification by solubilizing macronutrients, fixing atmospheric nitrogen, synthesizing phytohormones, siderophores, and ammonia which acting as a biocontrol agent. In this study, B. thuringiensis, B. paranthracis, S. xylosus, and B. cereus were isolated from leaves of Kalanchoe pinnata (Lam.) and identified with 16srRNA sequencing and submitted sequence in GenBank. All the isolated endophytes were positive to ammonia production and nitrogenase activity. The nitrogen quantity was found maximum in S. xylosus and B. paranthracis whereas minimum amount in B. thuringiensis and B. cereus. Amplified nifH gene was present in the ranges of 550bp to 580bp in isolated endophytes. These endophytes shown nitrogen fixing ability and growth performance was carried out with Zea mays. The isolates were shown improved shoot and root growth, appearance of flower and corn were early compared to control through its biological nitrogen fixation. These endophytes were used to fix nitrogen sustainably in agricultural crops and proved to an excellent alternative, eco-friendly fertilizer rather than chemical fertilizer for the benefits of farmers. Further evaluation of phosphate solubilization activity, siderophore production and plant growth promoting activity of these endophytic bacteria will credit their benefits in employing as efficient candidates in effective agricultural practices.
Acknowledgement
Authors expressed their acknowledgement to DBT Star College, New Delhi for support to carry out the work.
Conflict of Interest
All the authors declares that no conflict of interest throughout the work.
Funding Sources
There was no fund received from any agencies.
References
- Puri A, Padda K. P, Chanway C. P. Nitrogen-fixation by endophytic bacteria in agricultural crops: recent advances. Nitrogen in agriculture. IntechOpen, London, GBR., 2018; 1:73-94.
CrossRef - Puri A, Padda K. P, Chanway C. P. Can naturally-occurring endophytic nitrogen-fixing bacteria of hybrid white spruce sustain boreal forest tree growth on extremely nutrient-poor soils?. Soil Biology and Biochemistry, 2020; 1:140:107642.
CrossRef - Guo D. J, Singh R. K, Singh P, Li D. P, Sharma A, Xing Y. X, Song X. P, Yang L. T, Li Y. R. Complete genome sequence of Enterobacter roggenkampii ED5, a nitrogen fixing plant growth promoting endophytic bacterium with biocontrol and stress tolerance properties, isolated from sugarcane root. Frontiers in microbiology, 2020; 11:580081.
CrossRef - Bradshaw M. J, Pane A. M. Field inoculations of nitrogen fixing endophytes on turfgrass. Physiological and Molecular Plant Pathology, 2020; 112:101557.
CrossRef - Sati P, Sharma E, Soni R, Dhyani P, Solanki A.C, Solanki M.K, Rai S, Malviya M.K. Bacterial endophytes as bioinoculant: microbial functions and applications toward sustainable farming. InMicrobial Endophytes and Plant Growth, 2023;167-181. Academic Press.
CrossRef - Zhang Q, Acuña J. J, Inostroza N. G, Mora M. L, Radic S, Sadowsky M. J, Jorquera M. A. Endophytic bacterial communities associated with roots and leaves of plants growing in Chilean extreme environments. Scientific reports, 2019; 9(1):4950.
CrossRef - Rana K..L, Kour D, Kaur T, Devi R, Yadav A. N, Yadav N, Dhaliwal H. S, Saxena A. K. Endophytic microbes: biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek, 2020; 113:1075-107.
CrossRef - Alishahi F, Alikhani H. A, Khoshkholgh-Sima N. A, Etesami H. Mining the roots of various species of the halophyte Suaeda for halotolerant nitrogen-fixing endophytic bacteria with the potential for promoting plant growth. International Microbiology, 2020:415-27.
CrossRef - Fernandes J, M, Cunha L. M, Azevedo E. P, Lourenço E.M, Fernandes-Pedrosa M.F, Zucolotto S.M. Kalanchoe laciniata and Bryophyllum pinnatum: an updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Revista Brasileira de Farmacognosia, 2019; 29(4):529-58.
CrossRef - Saldierna Guzmán J. P, Nguyen K, Hart S. C. Simple methods to remove microbes from leaf surfaces. Journal of basic microbiology, 2020; 60(8):730-4.
CrossRef - Nxumalo C. I, Ngidi L. S, Shandu J. S, Maliehe T. S. Isolation of endophytic bacteria from the leaves of Anredera cordifolia CIX1 for metabolites and their biological activities. BMC Complementary Medicine and Therapies, 2020; 20(1):1-1.
CrossRef - Ramalashmi K, Prasanna V. K, Magesh K, Sanjana R, Siril J. S, Ravibalan K. A potential surface sterilization technique and culture media for the isolation of endophytic bacteria from Acalypha indica and its antibacterial activity. J Med Plant Stud, 2018; 181-4.
- Bergey, D. H. and Holt, J. G. Bergey’s manual of determinative bacteriology, 9th Ed, 2000; Lippincott Williams & Wilkins, Philadelphia.
- Wright M. H, Adelskov J, Greene A. C. Bacterial DNA extraction using individual enzymes and phenol/chloroform separation. Journal of microbiology & biology education, 2017; 18(2):10-128.
CrossRef - Wu T, Xu J, Liu J, Guo W. H, Li X. B, Xia J. B, Xie W. J, Yao Z. G, Zhang Y. M, Wang R. Q. Characterization and initial application of endophytic Bacillus safensis strain ZY16 for improving phytoremediation of oil-contaminated saline soils. Frontiers in Microbiology, 2019; 10:991.
CrossRef - Suhandono S, Kusumawardhani M. K, Aditiawati P. Isolation and molecular identification of endophytic bacteria from Rambutan fruits (Nephelium lappaceum L.) cultivar Binjai. HAYATI Journal of Biosciences, 2016 ;23(1):39-44.
CrossRef - Ahmad F, Ahmad I, Khan M. S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiological research, 2008 ;163(2):173-81.
CrossRef - Borah A, Das R, Mazumdar R, Thakur D. Culturable endophytic bacteria of Camellia species endowed with plant growth promoting characteristics. Journal of applied microbiology, 2019; 127(3):825-44.
CrossRef - Hongrittipun P, Youpensuk S, Rerkasem B. Screening of nitrogen fixing endophytic bacteria in Oryza sativa L. Journal of Agricultural Science, 2014 ;6(6):66.
CrossRef - Soper F. M, Simon C, Jauss V. Measuring nitrogen fixation by the acetylene reduction assay (ARA): is 3 the magic ratio?. Biogeochemistry, 2021 ;152(2):345-51.
CrossRef - Senthilkumar M, Amaresan N, Sankaranarayanan A, Senthilkumar M, Amaresan N, Sankaranarayanan A. Quantitative estimation of nitrogenase activity: acetylene reduction assay, 2021; 37-39.
CrossRef - Kjeldahl J. A method for determining nitrogen in organic materials. Z. Anal. Chem, 1883; 22:366.
CrossRef - Sulistiyani T. R, Meliah S. Isolation and characterization of nitrogen fixing endophytic bacteria associated with sweet sorghum (Sorghum bicolor). InProceedings The SATREPS Conference, 2017; 30 (1): 110-117.
- Gantait S, Kundu S, Das P. K. Acacia: An exclusive survey on in vitro propagation. Journal of the Saudi Society of Agricultural Sciences, 2018; 17(2):163-77.
CrossRef - Mokhichekhra S, Maftuna T, Bekhruz T. Search and Isolation of Endophytic Bacteria from Medicinal Plants and Determination of Their Morphological and Cultural Properties. Eurasian Journal of Research, Development and Innovation, 2021; 3:23-25.
- Sarjono P. R, Hazrina Q. H, Saputra A, Mulyani N. S, Aminin A. L, Ngadiwiyana N, Ismiyarto I, Kusrini D, Prasetya N. B. Isolation, characterization, and identification of endophytic bacteria by 16S rRNA partial sequencing technique from leaves of carica papaya and its potential as an antioxidant. InAIP Conference Proceedings, 2020; l(1): 2237. AIP Publishing.
CrossRef - Chaturvedi H, Singh V, Gupta G. Potential of bacterial endophytes as plant growth promoting factors. J Plant Pathol Microbiol, 2016; 7(9):1-6.
CrossRef - Husseiny S, Dishisha T, Soliman H. A, Adeleke R, Raslan M. Characterization of growth promoting bacterial endophytes isolated from Artemisia annua L. South African Journal of Botany, 2021;143:238-47.
CrossRef - Kang S. M, Bilal S, Shahzad R, Kim Y. N, Park C. W, Lee K. E, Lee J. R, Lee I. J. Effect of ammonia and indole-3-acetic acid producing endophytic Klebsiella pneumoniae YNA12 as a bio-herbicide for weed inhibition: special reference with evening primroses. Plants, 2020; 9(6):761.
CrossRef - Haidar B, Ferdous M, Fatema B, Ferdous A S, Islam M. R, Khan H. Population diversity of bacterial endophytes from jute (Corchorus olitorius) and evaluation of their potential role as bioinoculants. Microbiological Research, 2018; 208:43-53.
CrossRef - Haskett T. L, Knights H. E, Jorrin B, Mendes M. D, Poole P. S. A simple in situ assay to assess plant-associative bacterial nitrogenase activity. Front Microbiol, 2021; 12:1598.
CrossRef - Hrynkiewicz K, Patz S, Ruppel S. Salicornia europaea L. as an underutilized saline-tolerant plant inhabited by endophytic diazotrophs. J. Adv. Res, 2019; 19:49-56.
CrossRef - Abd Allah E. F, Alqarawi A. A, Hashem A, Radhakrishnan R, Al-Huqail A. A, Al-Otibi F. O, Malik J. A, Alharbi R. I, Egamberdieva D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact, 2018; 3(1):37-44.
CrossRef - da Silveira A. P, Iório R .D, Marcos F. C, Fernandes A. O, de Souza S. A, Kuramae E .E, Cipriano M. A. Exploitation of new endophytic bacteria and their ability to promote sugarcane growth and nitrogen nutrition. Antonie van leeuwenhoek, 2019; 112(2):283-95.
CrossRef - Singh R. K, Singh P, Sharma A, Guo D. J, Upadhyay S. K, Song Q. Q, Verma K. K, Li D. P, Malviya M. K, Song X. P, Yang L. T. Unraveling Nitrogen Fixing Potential of Endophytic Diazotrophs of Different Saccharum Species for Sustainable Sugarcane Growth. Int. J. Mol. Sci, 2022;23(11):6242.
CrossRef - .Argandoña M, Fernández-Carazo R, Llamas I, Martínez-Checa F, Caba J. M, Quesada E, Moral A. D. The moderately halophilic bacterium Halomonas maura is a free-living diazotroph. FEMS Microbiol. Lett 2005; 244(1):69-74.
CrossRef - Egamberdieva D, Wirth S. J, Shurigin V. V, Hashem A, & Abd_Allah E. F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Frontiers in Microbiology, 2017; 8, 1887.
CrossRef - Krishnamoorthy A, Agarwal T., Kotamreddy J. N. R., Bhattacharya R, Mitra A, Maiti T. K, & Maiti M. K. Impact of seed-transmitted endophytic bacteria on intra-and inter-cultivar plant growth promotion modulated by certain sets of metabolites in rice crop. Microbiological research, 2020; 241, 126582.
CrossRef - Fouda A, Eid A. M, Elsaied A, El-Belely E. F, Barghoth M. G, Azab E, … & Hassan S. E. D. Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (Lam.) DC inherent to arid regions. Plants, 2021; 10(1), 76.
CrossRef - Archana T, Rajendran L, Manoranjitham S. K, Santhana Krishnan, V. P, Paramasivan, M, & Karthikeyan G. Culture-dependent analysis of seed bacterial endophyte, Pseudomonas spp. EGN 1 against the stem rot disease (Sclerotium rolfsii Sacc.) in groundnut. Egyptian Journal of Biological Pest Control, 2020; 30(1), 1-13.
CrossRef - Wang S, Wang J, Zhou Y, Huang Y, & Tang, X. Prospecting the plant growth–promoting activities of endophytic bacteria Franconibacter sp. YSD YN2 isolated from Cyperus esculentus L. var. sativus leaves. Annals of Microbiology, 72022; 2(1), 1-15.
- Hagaggi N. S. A, & Mohamed A. A. Enhancement of Zea mays (L.) growth performance using indole acetic acid producing endophyte Mixta theicola isolated from Solenostemma argel (Hayne). South African Journal of Botany, 2020; 134, 64-71
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





