How to Cite | Publication History | PlumX Article Matrix
Laxmi Bhatti , Meena Bagiyal
, Meena Bagiyal and Sunita Khatak*
and Sunita Khatak*
Department of Biotechnology, University Institute of Engineering and Technology, Kurukshetra University, Kurukshetra-136119, Haryana, India.
Corresponding Author E-mail: sunitakhatak2019@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3208
ABSTRACT: Zinc oxide nanoparticles were synthesized using leaves of Tejpatta (Cinnamomum tamala) readily available as local herb in India. First confirmation was made by change of color of plant extract into cream color solution for zinc nanoparticle synthesis. The plant used as capping and reducing agent showed absorption peak of 329.4nm for zinc nanoparticle. While FESEM analysis further validated the nature of nano-particle synthesized of tetrahedral and crystalline zinc nanoparticles. The plant parts can be exploited for drug development as the plant is available in plenty growing all over arid region. Nanobiotech has served in recent years and utilized natural polymers or polymeric films, which have overcome the side effects of synthetic packaging films available commercially. The safety assessment should be in agreement with scientific advisory committee before permitting to packaging industries. The texture, flavor, processing, shelf life and transport of foods will become economical to meet the demand of food safety for future generations. Generally regarded as safe, packaging materials can be considered for globalization and revolution in food packaging sector. The present investigation reports zinc nanoparticle synthesis where potent zone of inhibition were reported against standard pathogen S. aureus and nanocomposite membranes were synthesized using different polymeric components available in reach like gelatin, sodium alginate and agarose instead of cellulose. The research will be further supplemented with application of biomembrane synthesized in antimicrobial resistance offered to food products.
KEYWORDS: Biomembrane; nanocomposite membranes; Packaging; spoilage; shelf life
Download this article as:| Copy the following to cite this article: Bhatti L, Bagiyal M, Khatak S. Zinc Nanoparticles and Nanocomposite Membrane Synthesized Using Leaves of Cinnamomum Tamala(Tejpatta) and Packaging Potential in Food Sector. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Bhatti L, Bagiyal M, Khatak S. Zinc Nanoparticles and Nanocomposite Membrane Synthesized Using Leaves of Cinnamomum Tamala(Tejpatta) and Packaging Potential in Food Sector. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/43qbLoS |
Introduction
The rate at which population is increasing day by day in especially developing countries is raising serious concern for food scarcity and sustainability. Moreover the public concern for quality food products, for health, well being is impelling food industries to be more precise in natural ingredients and quality related attributes of food for big market without compromising the nutritional content. Nanomaterial in the past ten years has extended its empire almost all sectors including essential food sector 1,2. Investors have shown keen interest in industries using advanced techniques like nanobiotechnology in food safety, pathogen detection, extended shelf life, food packaging and maintaining essential quality nutrients having consumer acceptability and need to get contamination free food products. Many researchers have revolutionized the food industrial sector with patents on a large number of safe food products developments and none the less nanotech served the sector to the maximum3. The major novel properties like strength, solubility, diffiusiblity, color and flavor have been modified and get enhanced owing to changed optical, thermodynamic and magnetic properties along with high durability, ductility4. Swami and co-associates in 2003reported that nanomaterial exploited for food industry is more stable at high temperature and pressure. Nanobiotech has already expanded its roots in food manufacturing and safety concerns.
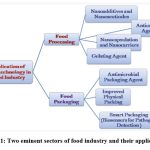 |
Figure 1: Two eminent sectors of food industry and their applications. |
Cinnamomum tamala belongs to family Lauraceae and commonly called as Indian bay leaf or tejpatta, Indian cassia, Malabar leaf or tejpat. The plant serves as multifunctional asset to Indian spices having immense potential in health sector. The bark and leaves are used in culinary purpose and in most of the holistic rituals in India. The left over leaves as plant material after rituals go wasted and picked up as being good source for many ailments cure. The aroma its leaves possess makes it excellent flavoring agent in medicinal sector along with food industries. There exist two sectors, food nanosensing and food nanostructural ingredients (Fig-1). Food nanosensing is mandatory for better food quality and food safety5. While food nanostructural ingredients extend from food processing to food packaging. Food processing involves uses of food additives, anti caking agents, carriers, antimicrobial agents while food packaging relates to nanomaterial as fillers which impart strength, durability to packaging material from moisture and heat 6. Nano-encapsulation comes under food processing. It enhances or savior of odor do not allow interaction with irrelevant ingredients only release at target site and at particular time being specific.
Some metallic nanoparticles in food sector
Silver NPS
Nanosilver composites have been developed by incorporating silver into polymers which in turn preserve food and increase its shelf life (Table-1). They have antimicrobial potential and have been practiced commercially. The size was in 20-70nm range found in 20 bulk polymers. AgNPs synthesized act against viruses, fungi, bacterial strain 7 . AgNPs target the cytoplasmic membrane, disrupts ETC chain and triggers ROS causing bacteriocidal activity by binding to DNA, protein and enzymes 8,9,10 . AgNPs incorporated films of sodium alginate were used in packaging of food products against E. coli. and S. aureus 11. Researchers reported that size is crucial factor which in turn dependent on temperature, concentration and active against number of standard pathogens P. aeruginosa,S. aureus, E. coli and C. albicans12,13,14,15 .
Titanium NPS
Also used as antimicrobial and whitening agent. These nanoparticles are layered structures incorporated in bulk polymers. The amount although needs to be below acceptable levels which is 0.001mg/L. Kumar and coworkers in 2021 reported antimicrobial activity of metallic nanoparticles, which are based on ROS, secondary metabolites and oxidizing cell components. The membrane disruption of microbial organism and interference in ETC chain in turn leads to cell death 16,17 .
CuNps
Were found significantly effective in inhibiting S. aureus, L. monocytogenes, E. coli and S. cerevesiaewhile polyurethane nano fibres having CuNps reported active against E. coli and B. subtilis18 .
Zinc NPS
They have immense potential mostly exploited in agriculture and food sector owing to antibacterial aspects to combat with new pandemic leading to resistance to most commonly used antibiotic prescribed. The ZnO metal inorganic oxide is active against a number of pathogens like S. aureus, E. coli, L. monocytogenes, C. jejuni and Salmonella spp.The derivatives of zinc and zinc itself are used as nanocomposites or membranes in food preservation. Researcher reported that zinc oxide inhibit bacterial cells (Zno with cellulose films) as nanocomposites and found to be active against S. aureus and E. coli.19 .Zinc nanoparticles incorporated plastic matrixes were found active against many fungal and bacterial strains.
Table 1: Application of Nanoparticles on the basis of its types.
| Type of Nanoparticle | Applications |
| Ag NP | Antimicrobial agent and Food packaging material |
| Zn NP | Source of Zn in supplements, functional foods, essential trace elements, food packaging and used for protection of UV Sensitive foods |
| Fe NP | Food coloring agents, mineral fortified supplements and functional foods. |
| TiO2 | Whitening agents and used in chewing gums. |
| SiO2 | Anti-caking Agents and enhance flow properties,Commonly used in salts, icing sugars, spices and dried milk. |
ZnO, MgO, TiO2 have photocatalytic disinfection properties and are also good UV blockers, TiO2 are active even under UV light radiations and poses inhibiting potential against S. choleraesuis,V. parahaemolyticus, L. monocytogenes in presence of light conditions20,21 by acting on oxidative entities on both side of cell membrane, causing DNA damage and altering Co-A activity.Further TiO2 has been reported against nine bacterial species. Erwinia caratovora, B. staerothermophilus, E. coli, S. aureus, Z. rouxii, L. plantarum, P. fluorescence, P. jadinii. TiO2 coated plastic films showed inhibition propensity against Penicillium expansum on the spoilage of lemon, apples and tomatoes (Table.2). ZnO act by destruction of cell wall integrity, resulting in ROS production and finally releasing Zn2+ions. ZnO also inhibit S. aureus based on reduced particle size and requires visible light for activation 22
Materials and Methods
Materials
Plant and culture collection
The leaves of medicinal plant Tejpatta (Cinnamommum tamala) selected in the present study were collected from Kurukshetra University, Kurukshetra, Haryana, India (Fig-2) and further identified from Botany Department of Kurukshetra University, Kurukshetra. The human pathogenic microorganism was procured from Microbial Type Culture Collection (MTCC): Institute of Microbial Technology (IMTECH), Chandigarh; which included Gram-positive bacteria: S. aureus (MTCC-3160).
Reagents and chemicals required
All the reagents and chemicals used in the present investigation were provided by Biotechnology Department of UIET, KUK purchased from Sigma chemical and Hi media. Sodium Alginate from Hi-Media (MB114), Glycerol purified (RM081), Formaldehyde solution 37% (MB059), used were of high analytical grade.
 |
Figure 2: Dry leaves of Tejpatta. |
Methods
Preparation of plant aqueous extract
After being collected and carefully handled to preserve their freshness, the plant parts—leaves included—were dried at room temperature and then ground into a powder. The 10g of powder was soaked in 100 ml of hot aqueous extracts and allowed to sit at room temperature for 72 hours. Using Whatman filter paper No. 1, the extracts were filtered. A water bath set at 45–50°C was used to evaporate the solvent. Following solvent extraction, DMSO was used to dissolve the leftover powder, which was then kept at 4°C23.
Preparation of ZnO nanoparticles
Approximately 6g of zinc nitrate (25mM) was dissolved in 90ml of triple deionized water and incubated at ambient temperature in water bath till solution become homogeneous. For a full hour, the 90 ml of this dissolved zinc solution was dropped dropwise into 10 ml of each plant extract from several medicinal herbs. The solution was then incubated in a water bath at 70°C. For an antimicrobial analysis, the prepared solution was examined in more detail.
Confirmation of Zn nanoparticles
Synthesized Zn nanoparticles were confirmed by visual observation via color change of original dark colored solution to cream colored solution and taking absorption maxima at the wavelength range of (300-600)nm 24 . The eco-friendly method of single step for zinc nanoparticle synthesis was used as reported earlier in our research articles with slight modifications.
Standardization of parameters using membrane synthesis
Various methodology and material were tried for membrane synthesis viz. Sodium alginate, Gelatin, Cellulose Acetate Butyrate and Agarose. Different concentrations, Temperature and incubation time, cross linking agent were opted for obtaining a membrane with better consistency.
Characterization of zinc nanoparticles and membrane
The characterization of nanoparticles and membrane was done using visual confirmation of color change from colorless to cream colored solution. It was further confirmed using UV-visible spectroscopy; XRD and FESEM analysis were performed using standard protocol from Department of Electronics and Communication of Kurukshetra University, Kurukshetra Haryana, India.
Antimicrobial analysis
Antimicrobial analyses of zinc nanoparticles with two fold dilution starting from 1mg/ml stock solution were prepared. The antimicrobial activity of nanocomposites membrane synthesized was tested against gram positive S. aureus using standard method given by 25 .
Results and Discussion
Nanotechnology is utilized in food packaging to increase food product safety26 . In the food industry, active packaging materials consist of nanoparticles made of titanium dioxide, silicon dioxide or even silver, which extends the shelf life of food products by changing their mechanical and heat resistant properties in addition to developing, antimicrobial and antifungal properties27,28 . Nanoparticles are utilized in enhanced packaging to alter the physical properties of food goods. It is used to protect food products from UV radiation. Nano biosensors are utilized in smart packaging to detect pathogenic activities029 . These biosensors are becoming more capable of responding to changes in the environment, such as temperature, oxygen level and humidity. They can also communicate and deliver information on food product degradation and microbiological contamination. They can also provide storage details as well as the time of storage of food products, eliminating the requirement for an expiry date label and providing consumers with exact fresh food. These biosensors also ensure the traceability of food and the safety of the product by detecting the situation of force during storage and transport in packaging. Nano layers of bio plastic are used in order to maintain the integrity of foods for the detection of food pathogens no fluorescent material is used it is manufactured without magnetic materials. Many food companies now employ nanotechnology in food processing. In the modern use of this technology, food products are processed in many ways before consumption, including heat treatment, fermentation and acid hydrolysis, drying, curing and smoking. In the food processing industry nanotechnology is used to improve the texture, integrity and flavor of the food by employing enzymes30 . They utilize various enzymes to increase the nutritional value of food, enhance the flavor of the meal and provide good health advantages to the customer. Nonmaterial are used in the food industry because they provide significant support to the enzyme system and improve product stability, reusability and adaptability. This technology is employed in a variety of industries for example some nanoparticles are used as anticaking agents in food products which improve uniformity and prevents lump formation31 .
Visual color change as first step of confirmation
The Zn nanoparticles were confirmed by visual color change of dark colored plant extract and colorless zinc nitrate solution to cream colored solution on reduction using plant leaf extracts and leaves as plant part offering dual benefit of capping and reducing effects (Fig-3).
 |
Figure 3: Color change as first confirmation of zinc nanoparticle synthesis from A) Tejpatta. |
UV visible spectroscopy:
The Zinc nanoparticle formation were confirmed by positioning of surface plasmon resonance in the UV spectroscopic analysis that shows the UV spectra of zinc nanoparticles synthesized by plant extracts that were exposed at different wavelength intervals. The maximum absorption peaks was observed at329.4nm which indicates the formation of zinc nanoparticles (Fig-4).The zinc nanoparticles formed are necessarily subjected to XRD analysis for the measurement of size(fig.4) shows the FESEM results obtained for the zinc nanoparticles synthesized using plant extract. The intense peak of nanoparticles appeared which are indexed as crystalline zinc. Tetrahedral nanoparticles were obtained as the sharpening of peak was observed. UV spectral analysis in the 300–400 nm range was used to further confirm the synthesis, and the results showed an optical absorption band peak at 328.8 nm.While UV visible spectrophotometer revealed peak at 329.4 nm confirming nanoparticle synthesis.
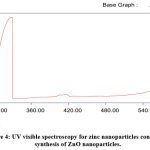 |
Figure 4: UV visible spectroscopy for zinc nanoparticles confirming synthesis of ZnO nanoparticles. |
FESEM results of zinc nanoparticles
FESEM analysis was conducted for both zinc nanoparticles and zinc incorporated sodium alginate membrane. The zinc nanoparticles resulted in irregular structures may be owing to agglomerates which further need calcinations to be separated simpler spherical nanoparticles as shown in below figures.
 |
Figure 5: FESEM results of zinc nanoparticles |
Antimicrobial efficacy of leaf extracts of Tejpatta mediated monometallic Zn nanoparticles
The zinc nanoparticles synthesized using leaves were used at initial concentration of 1mg/ml and serial wise 2 fold dilution was done till last concentration as explained in previous reports that any antimicrobial agent is effective given the size of inhibition zone, produced measures2mm or more. One volumes of extract (100µl) were tested against single standard pathogens S. aureus to test the efficacy, significant zone of inhibition were observed as shown all volumes of extracts. The nanoparticles resulted in zones of inhibition of size of18mm (S. aureus) at 100µl using nanoparticles.
Standardization of different parameters for biomembrane synthesis
Two gram sodium alginate powder was dissolved in 25ml Deionized water and incubated for thirty minutes at 70°C. Glycerol 12% (w/w) as a plasticizer is added in the film-forming solution (FFS) and tested for better consistency at a range of temperature ranging from 45-50°C to avoid lump formation in biofilm formation. Zinc (Zn) nanoparticles at different concentration with 1% formaldehyde as Cross-linking agent were added. Again the temperature is raised to 70°C and the solution is stirred well. Mixing is done for 30 minutes on a hot plate magnetic stirrer. Pour 25ml of this solution in petri-plates and dried at room temperature for 48-60 hours before peeling off and obtain a god consistent membrane. (Table-3)
Table 2: Concentration of different sample used for nanocomposites preparation before the Nanoparticles immobilization.
| Sr. No. | Sample name and Conc. (in gm) for 100ml | Diluted with and conc. (in ml) | Water bath and Duration | Plasticizer agent used and %age | Cross-linking agent used and %age | Time used for stirring in Magnetic stirrer | Settling time & temperature before peeling | Output |
| 1. | Gelatin-2g | Distilled H2O-25ml | 30 minutes at 70°C | Glycerol-12% | Formaldehyde-1% | 30 minutes at 70°C | Dried at room temperature for 36-48 hours. | Prepared well but pores are not homogenous. |
| 2. | Sodium Alginate-4g | Distilled H2O-4g | 30 minutes at 70°C | Glycerol-12% | Formaldehyde-1% | 30 minutes at 70°C | Dried at room temperature for 48-60 hours. | Prepared very good with homogenous pores. Hence, considered for further analysis |
| 3. | Cellulose Acetate Butyrate-3.75g | Acetone-25ml | Nil | DMSO-12% | Nil | 30 minutes at 50°C | Instant drying and peeling. | Prepared messy |
| 4. | Cellulose Acetate Butyrate-3g | DMSO-20ml | Nil | DMSO | Nil | 5 hours at room temperature | Instant drying and peeling. | Prepared average but pore size is not homogenous |
| Note: In No.1 and No.2 sample, Distilled H2O is heated at 70°C and cool until temperature reaches in the range of 45-50°C and then add Plasticizer and Cross-linking agent. | ||||||||
Table 3: Record and information taken from X-RD analysis and the sample no. are same as per characterization of sample and mentioned in above Table No. 3.
| Sample No. | 2ɵ | FWHM | Wavelength
(average) |
Result | Average Crystalline size |
| 1. | 21.2323
27.4832 63.0867 70.2222 76.3639 |
1.8893
0.4723 0.3149 0.4723 0.48 |
0.15418 | 4.47nm
18.09nm 30.93nm 21.49nm 22.00nm |
19.396nm |
| 2. | 20.6826
22.3816 26.7038 28.4582 44.4946 58.6204 |
1.5744
0.4723 0.4723 0.4723 0.3936 0.384 |
0.15418 | 5.36nm
17.92nm 18.06nm 18.13nm 22.79nm 24.79nm |
17.8416nm |
| 3. | 19.8094
25.76 29.0075 47.5807 71.3289 |
1.8893
0.4723 0.6298 0.7872 0.48 |
0.15418 | 4.46nm
18.03nm 11.75nm 11.52nm 21.29nm |
13.41nm |
| 4. | 20.9529
25.3891 47.1258 |
1.8893
0.3936 6.144 |
0.15418 | 4.47nm
21.62nm 1.47nm |
9.186nm |
Note: Wavelength is taken as average wavelength used in X-RD analysis technique. Result is calculated by the calculator given in the link: XRD d value Calculator- InstaNANO.https://instanano.com/all/ characterization/xrd/d-value/
XRD Analysis of Membrane Synthesized without NPS
XRD analysis- The phase structure and identification of the biologically synthesized nanoparticles (powder) were characterized by X-ray diffraction (XRD). The Cu 𝛼 radiation (𝑘 =1.5418 Å) was used to obtain the diffractogram in the range of 20-80 degrees 2θ. The average crystal size (D) of the nanoparticles was calculated using Debye-Scherer’s equation:
D = (0.94λ) / (β cos θ)
where λ is X-ray wavelength (λ = 1.5406 Å), θ is Bragg’s angle (2θ), and β is full-width at half-maximum (FWHM) in radians. X-ray diffraction (XRD) analysis was conducted using a PANalyticalX’Pert Pro instrument manufactured in Netherlands, at Department of Electronics, Kurukshetra University. The identification of compounds was carried out by comparing the results with standards in the Joint Committee of Powder Diffraction Standards issue 2010 (IUPAC 2010). XRD analysis was carried out for centrifuged, dried powder form of nanoparticles using tamala plant leaf extracts. The crystallinity and shape structure is elucidated for nanoparticles. The Zn nanoparticles were further utilized to synthesized nanocomposite membrane by standardizing different parameter using Alginate, Gelatin, cellulose acetyl butyrate 3.75g, cellulose acetyl butyrate 3g . The biomembrane characterized using XRD for all four combination. Pattern revealed of diffraction peaks at 2 theta such (20.6826, 22.3816, 26.7038, 28.4582, 44.4946, 58.6204) while the spectrum showed distinct separate peaks. The alginate based membrane was selected on the basis of having minimum difference between crystalline size of nanoparticles obtained giving a average size of 17.8nm. The nanoparticles formed via green approach are crystalline in nature. Other unknown peaks of XRD pattern showed presence of an organic compound in the extract. Average size of nanoparticles from XRD comes out to be 17.8nm as calculated by Debye Scherer’s equation.
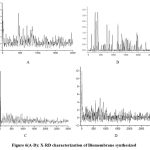 |
Figure 6:(a-d). X-RD characterization of Biomembrane synthesized |
The X-RD analysis of Sample No.2 membrane prepared by sodium alginate is considered as there is least number of differences in between two pores and showed the best size of pores in contrast with other membranes Table-4,(Fig-6, A-D).
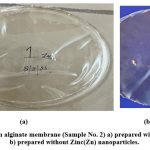 |
Figure 7: Sodium alginate membrane (Sample No. 2) a) prepared with nanoparticles b)prepared without Zinc(Zn) nanoparticles. |
FESEM results of biomembrane synthesized using zinc nanoparticles
FESEM results depicted the porous membrane with nanoparticle and did revealed consistent pore size. Zinc incorporated membrane have been synthesized earlier by many researchers where the membrane resulted in better packaging ,storage and increased shelf life and antibacterial activity of food products like orange juices mozzarell cheese, perishable fruits like green and black grapes, smoked fishes like salmon, Otolithesruber, Shrimps in addition to sliced wheat bread, chicken breast meat. The food showed antibacterial activity against n number of standard and food borne pathogens like L. monocytogenes and Salmonella typhimurium, E. coli, Salmonella enterica, Pseudomonas aeruginosa, Bacillus cereus and, and S. aureus. increasing shelf life from 9 days to 72 days and enhanced level of mechanical, thermal, and UV-protective properties32,33,34,35,36. The zinc nanoparticles incorporated ranges from a minimum of 0.5 % to a maximum of 20%. The spherical, hexagonal and polyhedral nanoparticles along-with their size ranging from 50 -100m have drastic effect on consistency and pore size of nano-membrane synthesized and also on bio-nanocomposite material used in investigation like done in present investigation as clearly depicted in figure below.
 |
Figure 8: FESEM of biomembrane synthesized using zinc nanoparticles |
Antimicrobial analysis of Zinc nanoparticles and Zinc nanocomposite membrane
Antimicrobial analysis was done using standard protocol given by Perez et al.,1990 with some modifications against standard pathogens bacterium S.aureus. The results show potent zone of inhibition as shown in fig-9.
 |
Figure 9: Antimicrobial activity of Zinc nanoparticlesand Zinc nanocomposite membrane against S. aureus. |
Zinc nanoparticles resulted in prominent zones on three volumes of extracts of 50,100 and 150 µl the zone size showed positive correlation to zone size.On increasing the volume of nanoparticle, the zone size increased as clearly shown in fig-9(a) while in comparison the membrane synthesized on incorporation of zinc nanoparticles in alginate resulted in smaller zone as compared to zinc nanoparticles in solution. A zone of 18,21,22,24 was observed as shown in fig-9(a),while membrane incorporated with zinc nanoparticles resulted in zone of 17mm only(fig-9(b).
Influence of nanoparticles on Food packaging sector
Packaging industry must work on permeability feature in packaging 37 . The gas and moisture must not be permeable and the material should be biodegradable after use. Nano composite have been in demand recently being non permeable, good mechanical strength, labeled with nanosensors to detect pathogens or deterioration of food after a certain period of time38 .Organic compounds cannot be utilized being susceptible to degrade at high temperature and pressure in polymeric matrices so inorganic nanoparticles are preferred being stable at adverse conditions and additionally equipped with antimicrobial resistance even at low concentration. These food particles are made resistant to microorganism by adding active nanoparticles which inhibit bacterial found on food surfaces. Zinc, titanium, silver copper nanoparticles are already incorporated in chitosan and available in market for antimicrobial properties. Nanotechnology which is the study of very small materials has the potential to drastically change the food industry. Due to its submicroscopic nature, nanotechnology has a lot of potential for modifying the color, flavor, and nutritional value of food, extending food’s shelf life and using barcodes to check food integrity in situations like the cold chain, or anytime there are even small changes in the conditions under which food is stored typically distinguished between two types of nano-food applications39 .
Nano-Packaging
Gas and moisture-permeable, biodegradable, and robust qualities are necessary in a packing material40 . A few benefits of nano-based “smart” and “active” food packaging over conventional packaging methods include better packaging materials with enhanced mechanical strength, barrier qualities, and antimicrobial films; additionally, nano sensing for pathogen detection and informing consumers of the safety status of food 41 .
Active Packaging
The usage of active nanomaterials such as antimicrobials and oxygen scavenging compounds is referred to as active packaging. The usage of such nanoparticles is advantageous for interacting directly with food to give improved product protection. Some nanoparticles have antibacterial capabilities that can be added to food packaging. Nano silver, nano-titanium dioxide, nano-magnesium oxide, nano-copper oxide, carbon nanotubes and other materials are examples. Active packaging makes use of packaging materials that interact with the environment and food, as well as playing an active role in enhancing product shelf life. These packaging methods include carbon dioxide, odor and ethylene absorbers as well as CO2 and scent emitters.
Smart/Intelligent Packaging
Smart packaging is intended to detect any microbiological or biochemical changes in food goods, as well as the growth of pathogens in food. Some smart packaging has been designed to be used as a food safety tracking device. Nanotechnology has been utilized to create “smart” packaging that can significantly extend the shelf life of food, allowing it to be carried over longer distances. The purpose of intelligent or smart packaging is to track and communicate food quality data. It includes radio frequency identification, biosensors, ripeness indicators, and TTIs. Such smart gadgets can be embedded into package materials or placed inside or outside of a container 42 . Currently, chemical sensors are used by Nestle, British Airways, and MonoPrix supermarket to quickly detect any color change43 .
Nanobiosensor detect pathogens in food samples have great implication in food microbiology. Food safety status and quantification of food constituents can be done using nanosensors. They act as indicator which responds with change in environment factors like temperature, humidity, moisture and pressure of store rooms. Ex-thin films, nano fibres, nanorods, can be employed as biosensors. Mostly they are used as optical immune-sensors which are thin film based and highly sensitive optical sensors have protein molecules immobilized on thin nano-biofilm or sensor chips, which emit signals on detection of Target molecule.Tan and his co associates in 2016 reported dimethylsiloxane microfluidic immunosensor is coupled with particular antibody immobilized on alumina nano-porous membrane for detection of E.coli and S.aureus with electrochemical impedence spectrum. Nanosensors can also detect pesticides, toxins, and pathogens. 44 and also reported carbon nanotubes based biosensor being economical, simpler and rapid in action in pathogen detection, toxic ingredients detection along with degraded products in beverages and food products. Logical studies have approved huge number of potential applications of zinc oxide nanoparticles such as antimicrobial impacts, astringent, anti-dysenteric, antipyretic, injury mending antidiarrheal, demulcent pain relieving insecticidal, photocatalytic and gastroprotective activities (Table-4)45 .
Table 4: Bacteria involved in number of potential ailments
| Bacterial meningitis | S. pneumoniae, S. agalactiae,
Listeria monocytogenes |
| Pneumonia | Mycoplasma pnuemoniae, S. pneumoniae, |
| Otitis media | S. pneumoniae, |
| Gastritis | Helicobacter pylori |
| Skin infections | P.aeruginosa, S. pneumoniae,
Staphylococcus pyrogenes |
| Sinusitis | Haemophilus influenza,S. pneumoniae, |
| Food poisoning | Staphylococcus aureus,E.coli |
| Urinary tract infection | S.saprophyticus,E.coli, P.aeruginosa, |
5.Migration – a serious concern for biofilm used in packaging
Nanocomposite synthesized using inorganic nanoparticles although acts as barriers to contaminants and result in enhanced antibacterial properties but along with have chances to migrate into food items which in turn further correlated to several factors like environmental (temperature, mechanical stress), Characteristics of nanomaterial (size, dispersion, shape, concentration), food state(pH, Composition, Constituents),Polymer properties(Structure, Viscosity, Contact time, duration) (Table-5)46 .
Table 5: Migration of nanoparticles in food and standard terms used.
| Migration | OML(Overall migration limit | Total amount of all nonvolatile substances which migrate to food | Most common in use | Permit 60mg/Kgof food in contact with polymer |
| SML(Specific migration limit) | Minimum amount of a particular substance present in food | Less common | Need detection assays | |
| QM(Maximum permitted quantity) | The degree of residual substances in food contact material |
Some researchers have advised the packaging sector for alarming health hazards of using nanocomposites in packaging material some are enlisted here(Table-6,7)
Table 6: Literature reported adverse impact of nano migration in food
| Sr no. | Characteristics of nanoparticles | Impact |
| 1. | Migration based on size, concentration, aggregation from packing material to food | Adverse impact on health |
| 2. | Being nano,micro scale | Can bio-accumulate within body organs and tissues |
| 3. | Silica nanoparticles in anti caking agents | Accumulate and cytotoxic to lung cells in humans |
| 4. | After (OML) concentration higher than recommended | Toxicity |
| 5. | Percentage of nanofillers in nanocomposites | More critical than migration, size,contact time, and temperature. |
Table 7: Harmful effects of nanomaterials
| Type of Nanomaterial | Possible harmful effects |
| Silver Nanoparticles | Elevation in cholesterol |
| C-60 Nanoparticles | Binds with RNA and DNA and damages them. |
| Copper Nanoparticles | Damage to liver, spleen and kidney. |
| Silicon Nanowires | Affected the activity of Taq Polymerase and restriction endonucleases. |
Migration is post packaging factor dependent, so standard protocols with strict labeling or instructions are not devised. So case study needs to explore the required standards for packing material. The process of migration have 50% chances of migration and totally unintentional when comes in contact with food items.EU, European regulation commission mentioned a 5-25mg Zinc/kg food as recommended amount of zinc allowed migrating in food items.NIH recommends 10mg/day zinc consumption for human body47 .This much migrated level is considered GRAS or say non toxic as specific concentration in food. So zinc concentrations in nanocomposite have been found to be below the max migration limit as reported in literature.
Conclusion
The technique makes available the technology to save food in biomembrane that increases the shelf life of food, avoiding spoilage and also making it bacterial and fungal resistant. The great attempts are being directed to standardize the nanocomposite membranes in which it can meet the anti-microbial packaging requirements. The nanocomposites can be synthesized based on various methods and procedures which can provide advanced membrane with different morphological and structural properties. We have successfully produced zinc based nanocomposites membrane by using sodium alginate with some modifications. The produced nanocomposites is a new effective absorbent that may find end uses in the field of packaging and heavy metal removing from contaminated and cleaning of environment. Zinc nanoparticles were synthesize using Cinnamomum tamala (Tejpatta) leaves the nanoparticles were confirmed by visual observation of change in colour from green plant extract solution to cream colour which was further validated by UV spectroscopy giving a peak at 329.4nm. FESEM analysis revealed a crystalline shape of nanoparticle. The Zn nanoparticles were further utilized to synthesized nanocomposite membrane by standardizing different parameter using Alginate, Gelatin, cellulose acetyl butyrate 3.75g, cellulose acetyl butyrate 3g . The biomembrane characterized using XRD for all four combination. The alginate based membrane was selected on the basis of having minimum difference between crystalline size of nanoparticles obtained giving a average size of 17.8nm. The FESEM analysis were also performed to check the membrane structure. The membrane can be used further in packaging industry for being biodegradable and being nontoxic. Then the food article can be preserved for longer time increasing shelf life and providing a potential in packaging sector. There are undoubtedly numerous ways to increase the use of immobilized nanoparticle-based nanocomposites for environmental processes, which deserve consideration in future works. Additionally, sodium alginate films were profoundly inhibited the antimicrobial activity against both Gram-negative and Gram-positive bacteria. Finally, strategies to regenerate nanocomposites to promote their recycling during their applications are also demanded. In addition, it is advised that sodium alginate films containing glycerol and nanoparticles be further investigated as active food packaging materials because they showed improved functional qualities and good compatibility. Our present investigation provides a biomembrane encrusted with zinc nanoparticles as a packaging option for food industry. The antimicrobial results clearly revealed the usage of these membranes in packing to be active against food-borne pathogens.
Acknowledgement
We are obliged to Department of Biotechnology, U.I.E.T. for providing Laboratory facility to carry out the project.
Conflict of Interest
There is no conflict of interest.
Funding Sources
The project was carried out in Department Laboratory and no financial assistance was obtained from anywhere.
References
- Jain A., Ranjan S., Dasgupta N. and RamalingamC. Nanomaterials in food and agriculture: an overview on their safety concerns and regulatory issues. Critical reviews in food science and nutrition, 2018; 58(2): 297-317.
CrossRef - , Shukla S., Kumar P., Wahla V., Bajpai V.K. and Rather I.A. Application of nanotechnology in food science: perception and overview. Frontiers in microbiology, 2017; 8:1501.
CrossRef - Dasgupta N., Ranjan S., Mundekkad D., RamalingamC., Shanker R. and Kumar A. Nanotechnology in agro-food: from field to plate. Food Research International, 2015;69: 381-400.
CrossRef - QureshiM. A., Karthikeyan S., Karthikeyan P., Khan P. A., Uprit S., & Mishra U. K. Application of nanotechnology in food and dairy processing: an overview. Pak J Food Sci,2012; 22(1): 23-31.
- Ezhilarasi P. N., Karthik P, Chhanwal N, andAnandharamakrishnan C. “Nanoencapsulation techniques for food bioactive components: a review.” Food and Bioprocess Technology, 2013; 6: 628-647.
CrossRef - Durán N., and Marcato P.D. Nanobiotechnology perspectives. Role of nanotechnology in the food industry: a review. International Journal of Food Science & Technology, 2013; 48(6): 1127-1134.
CrossRef - Duncan T.V. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. Journal of colloid and interface science, 2011; 363(1): 1-24.
CrossRef - Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T. and Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology, 2005; 16(10): 2346.
CrossRef - Emamifar A. Applications of antimicrobial polymer nanocomposites in food packaging. Advances in nanocomposite technology, 2011; 299-318.
CrossRef - Cavaliere E., De Cesari S., Landini G., Riccobono E., Pallecchi L., Rossolini G.M., and Gavioli L. Highly bactericidal Ag nanoparticle films obtained by cluster beam deposition. Nanomedicine: Nanotechnology, Biology and Medicine, 2015; 11(6):1417-1423.
CrossRef - Fayaz M., A., BalajiK., Girilal M., Kalaichelvan P.T. and Venkatesan. Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. Journal of agricultural and food chemistry, 2009; 57(14): 6246-6252.
CrossRef - Rhim J.W., Wang L.F., LeeY. and Hong S.I. Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: laser ablation method. Carbohydrate Polymers, 2014; 103: 456-465.
CrossRef - Lin S., Chen L., Huang L., Cao S., Luo X. and Liu K. Novel antimicrobial chitosan–cellulose composite films bioconjugated with silver nanoparticles. Industrial Crops and Products, 2015;70: 395-403.
CrossRef - Nanocomposite materials for food packaging applications: characterization and safety evaluation. Food engineering reviews, 2016; 8(1): 35-51.
CrossRef - Cobos M., De-La-Pinta I., Quindós G., FernándezM.J. and Fernández M.D. Graphene oxide–silver nanoparticle nanohybrids: Synthesis, characterization, and antimicrobial properties. Nanomaterials, 2020; 10(2):376.
CrossRef - Tankhiwale R. and Bajpai S.K. Preparation, characterization and antibacterial applications of ZnO-nanoparticles coated polyethylene films for food packaging. Colloids and surfaces B: Biointerfaces, 2012; 90:16-20.
CrossRef - Chaudhary P., Fatima, F. and Kumar, A. Relevance of nanomaterials in food packaging and its advanced future prospects. Journal of inorganic and organometallic polymers and materials, 2020; 30:5180-5192.
CrossRef - Sheikh A., SoniK. and Lakshmi N. Study of ZnO-SnO2 nanocomposites: Structural, topological and electrochemical properties. In AIP Conference Proceedings, 2020; 2220(1).
CrossRef - , Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., BakhoriS.K.M., Hasan H. and Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-micro letters, 2015;7: 219-242.
CrossRef - Robertson G.L. Packaging and food and beverage shelf life. In The stability and shelf life of food, Woodhead Publishing. 2016: 77-106.
CrossRef - Maneerat C. and Hayata Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. International journal of food microbiology, 2006; 107(2): 99-103.
CrossRef - Jones N., Ray B., Ranjit K.T. and Manna A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS microbiology letters, 2008; 279(1): 71-76.
CrossRef - , RajendranA., & MeenakshisundaramM.2014. Green synthesis of zinc oxide nanoparticles using flower extract cassia auriculata. Journal of NanoScience and NanoTechnology, 2014; 2(1): 41-45.
- Minal S.P. and Prakash S. Cu-Zn and Ag-Cu bimetallic nanoparticles as larvicide to control malaria parasite vector: a comparative analysis. In 2016 IEEE region 10 Humanitarian technology conference (R10-HTC)2016: 1-6. IEEE.
CrossRef - Perez C., Pauli M. and Bazerque P. An Antibiotic Assay by Agar Well Diffusion Method. Acta Biologiae et Medicinae Experimentalis, 1990; 15: 113-115.
- Berekaa, M.M. Nanotechnology in food industry; advances in food processing, packaging and food safety. J. Curr. Microbiol. App. Sci, 2015; 4(5): 345-357.
- Chellaram C., Murugaboopathi G., John A.A., SivakumarR., Ganesan S., Krithika S. and Priya G. Significance of nanotechnology in food industry. APCBEE procedia, 2014; 8: 109-113.
CrossRef - Hoseinnejad M., Jafari S.M. and Katouzian I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Critical reviews in microbiology, 2018; 44(2): 161-181.
CrossRef - Bumbudsanpharoke N. and Ko S. Nano‐food packaging: an overview of market, migration research, and safety regulations. Journal of food science, 2015; 80(5): R910-R923.
CrossRef - He X. and Hwang H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. journal of food and drug analysis, 2016; 24(4): 671-681.
CrossRef - Daneshniya M., Maleki M.H., Amini F., Behrouzian M. and Latifi Z. March. Positive and negative aspects of nanocomposites utilization in food packaging. In 3rd International Congress of Science, Engineering and Technology2020:
- Jafarzadeh S.; Rhim J. W.; Alias A. K.; AriffinF.; MahmudS. Application of Antimicrobial Active Packaging Film Made of Semolina Flour, Nano Zinc Oxide and Nano-kaolin to Maintain the Quality of Low-moisture Mozzarella Cheese during Low-temperature Storage. J. Sci. Food Agri.,2019; 99(6): 2716–2725. DOI: 10.1002/jsfa.9439.
CrossRef - Kumar S.; Boro J. C.; Ray D.; Mukherjee A.; Dutta J. Bionanocomposite Films of Agar Incorporated with ZnO Nanoparticles as an Active Packaging Material for Shelf Life Extension of Green Grape. Heliyon. 2019; 5(6): 01867. DOI: 10.1016/j.Heliyon..e01867.
CrossRef - Baek S. K.; Song K. B. Development of Gracilariavermiculophylla Extract Films Containing Zinc Oxide Nanoparticles and Their Application in Smoked Salmon Packaging. LWT, 2018;89: 69–275. DOI: 10.1016/j.lwt.2017.10.064.
CrossRef - Indumathi M. P.; Rajarajeswari G. R. Mahua Oil-based Polyurethane/chitosan/nano ZnO Composite Films for Biodegradable Food Packaging Applications. J. Biol. Macromol., 2019; 124: 163–174. DOI: 10.1016/j.ijbiomac.
CrossRef - ; GhanbarzadehB.; Mokarram R.R.; HashemiM. Novel Active Packaging Based on Carboxymethyl cellulose-chitosan-ZnO NPs Nanocomposite for Increasing the Shelf Life of Bread. Food Packag. Shelf Life., 2017; 11: 106–114. DOI: 10.1016/j.fpsl.2017.01.010.
CrossRef - Couch L.M., Wien M., Brown J.L. and Davidson P. Food nanotechnology: proposed uses, safety concerns and regulations. Food Ind. Hitech, 2016; 27: 36-39.
- Pinto R.J., Daina S., Sadocco P., Neto C.P. and Trindade T. Antibacterial activity of nanocomposites of copper and cellulose. BioMed research international, 2013.
CrossRef - Zhang T., Lv C., Chen L., Bai G., Zhao G. and Xu C. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Research International, 2014; 62: 183-192.
CrossRef - Renton A. Welcome to the world of nano foods. The Observer, Guardian Unlimited, 2006; 16.
- Weiss J., Takhistov P. and McClements D.J. Functional materials in food nanotechnology. Journal of food science,2006; 71(9): R107-R116.
CrossRef - Mihindukulasuriya S.D.F. and Lim L.T. Nanotechnology development in food packaging: A review. Trends in Food Science & Technology, 2014; 40(2): 149-167.
CrossRef - Ameta S.K., Rai A.K., Hiran D., Ameta R. and Ameta S.C. Use of nanomaterials in food science. Biogenic nano-particles and their use in agro-ecosystems, 2020: 457-488.
CrossRef; - Nachay K. Analyzing nanotechnology. Food technology (Chicago), 2007; 61(1): 34-36.
- Primožic M., Knez Ž. and Leitgeb M. Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021; 11: 292.
CrossRef - McClements D.J. and Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. npj Science of Food, 2017; 1(1): 6.
CrossRef - Kumar S., Boro J.C., Ray D., Mukherjee A. and Dutta J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon, 2019; 5(6).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





