How to Cite | Publication History | PlumX Article Matrix
Managing Chemotherapy-Induced Diarrhea: Efficacy of Interventions for Cancer Patients
N. Venkateswaramurthy* , Aravindhan S
, Aravindhan S and Elavarasan P R
and Elavarasan P R
Department of Pharmacy Practice, J. K. K. Nattraja College of Pharmacy, Kumarapalayam, Tamil Nadu, India.
Corresponding Author E-mail: nvmurthi@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3233
ABSTRACT: Non-communicable diseases (NCDs) account for 71% of all deaths worldwide, with cancer being one of the leading causes of mortality in India (9%), where NCDs account for 63% of all fatalities. The incidence of cancer continues to rise, with breast, lung, colon and rectum, prostate, non-melanoma skin cancer, and stomach cancer being the most commonly diagnosed in 2020. Similarly, lung, colon and rectum, liver, stomach, and breast cancer are the most common causes of cancer-related deaths. Chemotherapy is widely used to treat cancer due to the rapid growth and reproduction rate of cancer cells. However, diarrhea is one of the most common side effects of chemotherapy. The management of chemotherapy-induced diarrhea involves a variety of pharmacological interventions, including loperamide and octreotide, as well as the use of probiotics and herbal products. This review provides a comprehensive overview of these treatments and their efficacy, as well as strategies for the prevention of chemotherapy-induced diarrhea
KEYWORDS: Cancer; Chemotherapy; Diarrhea; Irinotecan; Loperamide
Download this article as:| Copy the following to cite this article: Venkateswaramurthy N, Aravindhan S, Elavarasan. P. R. Managing Chemotherapy-Induced Diarrhea: Efficacy of Interventions for Cancer Patients. Biotech Res Asia 2024;21(2). |
| Copy the following to cite this URL: Venkateswaramurthy N, Aravindhan S, Elavarasan. P. R. Managing Chemotherapy-Induced Diarrhea: Efficacy of Interventions for Cancer Patients. Biotech Res Asia 2024;21(2). Available from: https://bit.ly/3JkMm6E |
Introduction
Cancer manifests through the unregulated proliferation of cells, which can spread beyond their original site in the body. Non-communicable diseases (NCDs) constitute a major global health issue, accounting for 71% of all mortalities.1 Cancer is one of the leading causes of death in India, contributing to 63% of all fatalities. Since 1982, Population-based Cancer Registries (PBCRs) and Hospital-Based Cancer Registries (HBCRs) in India have systematically collected data on cancer as part of the Indian Council of Medical Research’s (ICMR) National Cancer Registry Programme (NCRP).2
Globally, cancer is the leading cause of mortality, with approximately 10 million people succumbing to it in 2020 alone. The most common types of cancer, in terms of new cases in 2020, were breast, lung, colon and rectum, prostate, non-melanoma skin cancer, and stomach cancer.3 According to the World Health Organization (WHO), ischemic heart disease, stroke, and cancer are the primary contributors to the majority of disability-adjusted life years (DALYs). Leukemia is the most common cancer in children under the age of 14, while breast cancer is predominant among individuals aged 15 to 49. Lung cancer is the most prevalent among those over 50.4,5
Chemotherapy
Chemotherapy is a prevalent form of medical treatment that utilizes drugs to target and destroy rapidly multiplying cells within the body. It’s primarily employed in the battle against cancer, given that cancer cells proliferate and spread at a rate surpassing that of normal body cells. Chemotherapy medications are available in various forms and can be administered either singly or in combination to combat different cancer types. Although chemotherapy stands as a potent cancer treatment, it may induce a range of side effects on the body. Some side effects are mild and manageable, while others may pose serious health risks. Common side effects of chemotherapy include nausea, vomiting, diarrhea, hair loss, loss of appetite, fatigue, fever, mouth sores, pain, constipation, easy bruising, and bleeding.6
Chemotherapy agents are powerful drugs designed to target cancer cells at specific stages of their growth cycle, known as the cell cycle. This cycle represents the sequence of events in which cells grow and make copies of themselves. Since cancer cells traverse this cycle more swiftly than normal cells, chemotherapy is particularly effective against these rapidly dividing entities.7 A thorough understanding of the action mechanisms of chemotherapy drugs, alongside their potential side effects, is crucial for optimizing cancer treatment and ensuring comprehensive patient care.
Chemotherapy-Induced Diarrhea and its mechanism
Diarrhea is a frequently encountered side effect of chemotherapy, with its prevalence and severity varying based on the specific chemotherapy regimen employed.8-11 Certain chemotherapy drugs, notably 5-fluorouracil (5-FU) and irinotecan are associated with incidence rates of diarrhea as high as 80%.10,11 This condition can lead to significant complications, including malnutrition, electrolyte imbalances, and immune suppression. Chemotherapeutic agents commonly linked to the occurrence of diarrhea encompass fluoropyrimidines (such as 5-FU and capecitabine), topoisomerase I inhibitors (like irinotecan and topotecan), and a range of other medications including cisplatin, docetaxel, oxaliplatin, and cytarabine.12-19
The pathogenesis of diarrhea can result from either reduced absorption of water in the intestines or increased secretion of water into the intestinal lumen. While most acute diarrhea cases are attributable to infections by pathogens, it’s useful to differentiate diarrhea into secretory and osmotic types when considering its underlying mechanisms. Secretory diarrhea occurs due to either impaired electrolyte absorption or the excessive secretion of electrolytes into the intestinal lumen by epithelial cells. This type of diarrhea is characterized by fecal fluid that is high in electrolytes, stemming from either the active secretion of electrolytes into the intestine or a failure in their absorption, which primarily contributes to the development of diarrhea.20
MOA
The mechanism of action for secretory diarrhea involves the activation of intracellular signaling molecules such as cAMP, cGMP, and intracellular calcium. These mediators play a crucial role in initiating secretory diarrhea by enhancing the active secretion of chloride ions from crypt cells and inhibiting the absorption of sodium chloride via neutral pathways. This process affects the ionic flow across cellular junctions, exacerbated by toxin-induced damage to tight junctions. Acute-onset secretory diarrhea is most commonly caused by bacterial infections in the gastrointestinal tract. These infections can lead to the production of toxins that directly damage the gut lining or stimulate the body to produce cytokines. These cytokines attract inflammatory cells, which further increase secretion by promoting the release of substances such as prostaglandins and platelet-activating factors. Although the coupled transport of sodium, glucose, and amino acids is mostly unaffected, the condition may occasionally lead to hyperplasia in the intestinal crypts.21,22
Osmotic diarrhea, on the other hand, arises from the consumption of poorly absorbed substances found in the diet or certain medications that exert an osmotic effect. In cases of osmotic diarrhea, the fecal fluid is characterized by a high concentration of ingested non-absorbable solutes, leading to a lower concentration of electrolytes. This type of diarrhea occurs when the osmotic balance is disrupted by these substances, drawing water into the intestinal lumen and resulting in an increase in stool volume and fluidity.
MOA
Chemotherapy can precipitate diarrhea by inflicting damage on the gastrointestinal (GI) tract’s lining, notably reducing the surface area available for nutrient and water absorption due to harm to the villi. Furthermore, chemotherapy can prompt rebound crypt hyperplasia, where immature crypt cells ascend to the tip of the villus, impairing the efficient absorption of water and leading to diarrhea. Changes in gut motility, induced by chemotherapy, also contribute to diarrhea by diminishing the time bowel contents spend in contact with the colon wall, which in turn decreases water absorption.
It’s important to note that the colon, unlike the small intestine, does not possess villi. In the colon, chloride and subsequently water absorption occur through the walls of the colonic crypts. Chemotherapy-induced damage to these crypts interferes with chloride absorption, causing an accumulation of water in the intestinal lumen and, consequently, diarrhea. This disruption highlights the complex interplay between chemotherapy’s effects on the GI tract’s structural integrity and function, leading to various pathways through which diarrhea can manifest.23
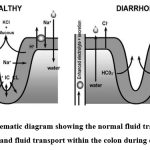 |
Figure 1: Schematic diagram showing the normal fluid transport within the colon and fluid transport within the colon during diarrhea 24Click here to view Figure |
Drugs that More Commonly Induce Diarrhea
Chemotherapy treatments are known to cause diarrhea, a side effect linked to the mechanism of action of these medications. Chemotherapy aims to eliminate rapidly dividing cells, such as cancer cells, but it can inadvertently affect healthy cells in the digestive tract, leading to diarrhea. Specifically, medications like 5-fluorouracil and irinotecan are notorious for inducing diarrhea. The severity of Chemotherapy-Induced Diarrhea (CID) is categorized into different grades. Grade 1 CID is characterized by mild diarrhea that does not significantly interfere with daily activities. In contrast, Grade 2 CID presents as more severe diarrhea that may require medical intervention for management. Loperamide is the recommended first-line treatment for managing Grade 2 CID. This grading system helps healthcare providers determine the severity of the side effects and the appropriate intervention needed to manage the patient’s symptoms effectively. By targeting cells that multiply quickly, chemotherapy medications play a crucial role in cancer treatment. However, their effect on the digestive system, particularly in causing diarrhea, highlights the delicate balance between treating cancer and managing the side effects of such powerful medications.
Irinotecan
Irinotecan plays a crucial role in the treatment of metastatic colorectal cancer, serving as a primary option in both first and second-line therapies.25-27 Despite its effectiveness, myelosuppression and delayed-onset diarrhea are among the most common side effects, occurring regardless of the dosing schedule.28 Acute diarrhea can manifest shortly after administration of irinotecan, attributed to its cholinergic effects, leading to symptoms such as abdominal cramps, rhinitis, lacrimation, and salivation. This acute phase typically lasts about 30 minutes, with atropine being an effective remedy.25 Delayed-type diarrhea, distinct from the acute phase, develops more than 24 hours after the administration of irinotecan. This form of diarrhea is non-cumulative and can occur independently of the dose. It is associated with several clinical predictors, including the weekly dosage, compromised performance status, increased serum creatinine levels, history of abdominal pelvic irradiation, reduced leukocyte counts, age over 70 years, Gilbert syndrome, and Crigler-Najjar syndrome type 1.29 The precise mechanisms behind irinotecan-induced diarrhea remain partially understood, but various theories have been proposed to explain its occurrence.
One prominent theory involves the metabolic processing of irinotecan’s active metabolite, 7-ethyl-10-hydroxy camptothecin (SN38), by the liver enzyme uridine diphosphate glucuronosyltransferase-1A1 (UDP-GT 1A1), resulting in the formation of SN38-glucuronide (SN38G). While SN-38 and SN-38G are excreted via bile and urine, fecal elimination is the primary route for disposing of irinotecan, accounting for 63.7% of the administered dose. In the intestinal lumen, bacterial ß-glucuronidase acts to deconjugate SN38G back into SN38. The presence of free SN38 in the intestinal tract, whether from bile secretion or the breakdown of SN38G, is believed to trigger the gastrointestinal side effects associated with irinotecan, including diarrhea.30-31
The mechanisms through which free SN38 induces diarrhea are multifaceted, with several theories proposed to elucidate this process. One theory posits that SN38 directly damages the intestinal mucosa in rats, leading to water and electrolyte loss as well as mucous hypersecretion.32 Another hypothesis suggests that irinotecan alters the intestinal microenvironment in a way that fosters the growth of specific bacterial genera. This altered environment enhances the activity of bacterial ß-glucuronidase, which then converts SN38G back into its active form, SN38, precipitating notable adverse effects, including diarrhea.33-34
Histological examinations of the gastrointestinal tract in rats after irinotecan treatment have revealed damage associated with the activity of ß-glucuronidase present in the gut lumen. The typical damage observed includes villous atrophy and crypt hypoplasia in the small intestine, alongside significant colonic damage characterized by increased apoptosis, crypt hypoplasia, and dilatation, all of which are accompanied by excessive mucous secretion. Variations in goblet cell populations, elevated apoptosis levels, histological alterations in the colon and jejunum, and disrupted absorption rates are all implicated in the onset of diarrhea.35-36,33 Additionally, irinotecan has been shown to markedly decrease mucin expression while significantly increasing mucin secretion in the rat colon and jejunum, as evidenced by immunohistochemistry analyses for Muc2 and Muc4. The link between increased mucin secretion and diarrhea suggests a connection to altered mucin gene expression.37 This intricate relationship underscores the need for further research to fully understand the pathophysiology of irinotecan-induced diarrhea and the role of mucin in this process.
Fluoropyrimidines (5-Fu, Capecitabine, Tegafur/Uracil)
Fluoropyrimidine-based chemotherapy, notably including agents like 5-fluorouracil (5-FU), is a cornerstone in the treatment of various cancers. Despite its efficacy, a significant side effect associated with this therapy is the development of diarrhea. The addition of Leucovorin (LV) to enhance the effectiveness of 5-FU treatment has been observed to increase both the frequency and severity of diarrhea induced by 5-FU.37 It is reported that around half of the patients receiving weekly combination therapy of 5-FU and LV experience diarrhea, with the condition exacerbating following bolus injections of 5-FU. Clinical observations have identified specific risk factors that elevate the likelihood of experiencing diarrhea during fluoropyrimidine treatment, including being female, Caucasian, or having a pre-existing condition like diabetes.
Despite the prevalence of diarrhea as a side effect, there is a notable gap in comprehensive research aimed at elucidating the exact pathogenic mechanisms behind it. Initial findings have suggested that 5-FU contributes to diarrhea by halting the division of intestinal crypt cells, leading to a decrease in villous enterocytes and subsequently reducing the intestinal surface area available for nutrient absorption. To deepen our understanding of the impact of 5-FU on the gastrointestinal tract and the onset of diarrhea, further studies have been conducted. These investigations involve varying the dosing regimens of 5-FU in animal models, aiming to pinpoint how this cytotoxic drug precipitates the development of diarrhea.38-39
Docetaxel
Docetaxel is an injectable chemotherapeutic agent widely used for the treatment of various solid tumors, including non-small cell lung cancer (NSCLC), breast, gastric, prostate, and head and neck cancer. Nevertheless, administration of docetaxel is linked with various undesirable outcomes such as fluid retention, neurosensory events, hair loss, skin-related issues, mouth inflammation, nausea, vomiting, diarrhea, and a decrease in white blood cell count (neutropenia). Diarrhea emerges as a prevalent side effect, impacting 20% to 40% of patients, while severe diarrhea affects approximately 5% to 6% of individuals undergoing treatment.40
Docetaxel-induced diarrhea’s pathophysiology involves the drug’s capacity to stabilize tubulin through binding, which impedes microtubule disassembly. Consequently, this action leads to cell cycle arrest at the G2/M phase, culminating in cell death.41
Anti-Egfr-Antibodies
The Epidermal Growth Factor Receptor (EGFR) has been extensively researched as a therapeutic target in various cancers due to its pivotal role in cell proliferation and differentiation. Anti-EGFR antibodies are employed to counteract the overactivation of the EGFR family, a key contributor to the pathogenesis of numerous human cancers. Members of the EGFR family are typically found as inactive monomers; they only become active upon ligand binding, which prompts the dimerization of two receptors. This dimerization triggers downstream signaling pathways that are vital for cancer cell growth and survival.
Despite the therapeutic benefits of targeting EGFR in cancer treatment, the administration of anti-EGFR antibodies is associated with several adverse effects, notably including diarrhea. The precise pathophysiological mechanisms underlying diarrhea induced by anti-EGFR therapy remain elusive. It is speculated that the disruption of EGFR signaling, which plays a significant role not only in tumor cells but also in the maintenance of normal intestinal mucosa, may contribute to this side effect. The inhibition of EGFR on intestinal epithelial cells could potentially alter cell turnover and repair, mucosal integrity, and fluid absorption, leading to the development of diarrhea. Further research is necessary to fully elucidate the mechanisms by which anti-EGFR antibodies induce this common and challenging side effect.42
EGFR tyrosine kinase inhibitors (TKIs)
Diarrhea associated with the use of EGFR tyrosine kinase inhibitors (TKIs) typically manifests within the first four weeks of treatment, with the highest likelihood of occurrence within the first seven days following the initiation of afatinib therapy.43 Several potential mechanisms have been proposed to explain the pathophysiology behind diarrhea induced by EGFR TKIs. A key hypothesis centers on the role of EGFR as a crucial negative regulator of chloride secretion in the gastrointestinal tract. In normal conditions, EGFR is often overexpressed in the healthy gastrointestinal mucosa, where it plays a significant role in maintaining fluid and electrolyte balance.
The administration of EGFR TKIs may disrupt this regulatory balance by inhibiting EGFR’s normal function. This disruption can lead to an unchecked increase in chloride secretion into the intestinal lumen, creating an osmotic gradient that draws water into the gut, thereby resulting in secretory diarrhea. This effect highlights the delicate balance maintained by EGFR signaling in the gastrointestinal tract and how its inhibition by TKIs can lead to adverse effects such as diarrhea.44
Table 1: Grade 3/4 diarrhea (CTC grades) rates for various medicinal substances and blends 45
| Agent | Grade 3/4 diarrhea | |
| Chemotherapy | Single-agent | Combination therapy |
| Irinotecan (late diarrhea) | 16–22% | 11–14% Folfiri (bolus/CI) |
| Docetaxel/paclitaxel | 4% | 14% docetaxel + capecitabine <br> 19% DCF |
| Anti-EGFR-antibodies | 1–2% | 15% cetuximab + Folfiri |
| 5-FU (bolus) | 32% (G3) | 26% Xeliri |
| 5-FU (CI) | 6–13% | 25–28% IFL (bolus) |
| Anti-EGFR-TKI | 6–9% | 13% lapatinib + capecitabine 15%lapatinib + paclitaxel 6%erlotinib + gemcitabine |
| Capecitabine | 11% | – |
| sorafenib/sunitinib | 2–8% (G3) | – |
| m- TOR inhibitors | 1–4% (G3) | – |
Management
Guidelines for Treating Chemotherapy-Induced Diarrhea. 79
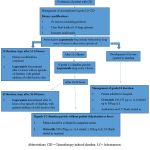 |
Chart 1: Flow ChartClick here to view Chart |
Pharmacological Management
Loperamide
Loperamide, an opioid derivative, primarily acts on the smooth muscles of the intestine to decrease bowel motility. Its systemic absorption is minimal, making it highly effective for reducing bowel movement frequency, lessening stool volume, and managing fecal incontinence. The initial recommended dosage of loperamide is 4 mg, followed by 2 mg every 4 hours or after each loose stool, with a maximum daily limit of 16 mg.46 Patients are advised to make dietary modifications and gradually reintroduce solid foods as diarrhea subsides with loperamide treatment. For cases of chemotherapy-induced diarrhea, it is recommended to discontinue loperamide once the patient has been free of diarrhea for at least 12 hours. If mild to severe diarrhea persists beyond 24 hours, the dosage may be increased to 2 mg every 2 hours, and oral antibiotics may be introduced to prevent infection.20 Acting as a non-analgesic agonist on opioid receptors within the myenteric plexus of the intestinal wall, loperamide effectively reduces intestinal motility and provides relief from chemotherapy-induced diarrhea, particularly at higher dosages. Nonetheless, it’s important to be cautious of its potential side effects, which can include severe constipation, abdominal discomfort, disorientation, skin rashes, and the exacerbation of pre-existing conditions like bloating, nausea, and vomiting. At elevated dosages, there is an associated risk of developing paralytic ileus and abdominal distension. Despite these risks, loperamide is widely recognized as the primary first-line therapy for managing chemotherapy-induced diarrhea, owing to its effectiveness.47-48,9,45,55
Deodorized Tincture of Opium (DTO)
Deodorized tincture of opium (DTO) is recognized as an antidiarrheal medication, often considered for the management of chemotherapy-induced diarrhea (CID), although comprehensive evidence supporting its use in this specific context is limited.45 Similar to loperamide, DTO acts by stimulating opioid receptors within the gastrointestinal (GI) tract. This action decreases peristalsis, extends the transit time, and promotes the reabsorption of fluids, effectively reducing diarrhea. Despite the scarcity of studies directly evaluating DTO’s efficacy in CID treatment, it is frequently employed as an antidiarrheal agent and may be considered a secondary option for the management of chronic and uncomplicated diarrhea.55 DTO contains a morphine concentration of 10 mg/mL, making it one of the strongest forms of orally administered morphine available by prescription. Its use can induce euphoria, along with potential side effects such as nausea, vomiting, urination difficulties, abdominal discomfort, seizures, and allergic reactions. Furthermore, DTO carries a risk of leading to psychological and physical dependence. Additional adverse effects include miosis (constricted pupils), respiratory depression (slowed breathing), and constipation. Notably, long-term opioid use is well-documented to cause severe constipation. Therefore, while DTO serves as an option for CID management, its potent nature and the possibility of significant side effects necessitate careful consideration and monitoring when prescribing it for diarrhea control.49,46,55
Octreotide
Octreotide, a synthetic analogue of somatostatin, exerts a broad spectrum of actions, including the suppression of various hormonal secretions such as vasoactive intestinal peptide (VIP). It effectively slows the transit of substances through the intestine, diminishes the secretion of fluids and electrolytes, and enhances their absorption. Approved by the FDA for mitigating the symptoms of carcinoid syndrome, particularly the diarrhea that occurs in association with tumors secreting VIP, octreotide also shows promise for patients suffering from chemotherapy-induced diarrhea (CID). This potential benefit extends to CID resulting from treatment with fluoropyrimidines, irinotecan, or 5-fluorouracil (5-FU)-based chemoradiotherapy.50-53
Octreotide is generally considered a second-line treatment for patients who do not experience relief from diarrhea after an increased dosage of loperamide over a 48-hour period. While the optimal dosing for octreotide remains to be definitively established, current recommendations suggest starting with an initial dose of 100-150 µg administered subcutaneously (SC) or intravenously (IV) three times daily. Adjustments such as continuous IV infusion or increasing the dose to 500 µg SC/IV three times a day may be considered based on patient response. Studies indicate a positive correlation between dosage and therapeutic response, with minimal adverse effects reported at infusion rates of 25-50 µg/hr. Despite its efficacy, the high cost of octreotide often restricts its widespread use in managing CID.54
Antibiotics
Recent guidelines recommend the initiation of fluoroquinolone antibiotics for complex and refractory diarrhea to prevent infectious complications that could escalate to sepsis. Fluoroquinolones, among other antibiotics, are also suggested when infectious diarrhea is suspected, offering a proactive approach to managing potential bacterial causes.55 For diarrhea attributed to pseudomembranous colitis, which is often caused by Clostridium difficile, metronidazole or vancomycin are the preferred treatment options.56,55 These antibiotics have been proven effective in addressing the underlying bacterial infection and alleviating the associated symptoms. Accurate diagnosis of the cause of diarrhea is essential to determine the most effective treatment strategy. In scenarios where infectious origins are suspected, the use of antibiotics, including fluoroquinolones, should be carefully considered and aligned with the latest clinical guidelines.55 The strategy of employing minimally absorbable antibiotics, such as neomycin, to target bacterial ß-glucuronidase in the intestine offers a novel approach to mitigating irinotecan-induced mucosal damage. This concept is based on the premise that the activation of SN-38G, a key factor in irinotecan-induced mucosal damage, depends on bacterial ß-glucuronidase activity.57 Although this approach has shown promise in some instances of secondary prophylaxis, the outcomes from a recent randomized phase II study—which observed a reduction in grade 3 diarrhea from 32.4% to 17.9%—failed to achieve statistical significance.58-59 Conversely, a separate nonrandomized study involving 51 patients treated with levofloxacin reported only one instance of grade 3 diarrhea, with no cases of grade 4 diarrhea observed. These divergent results underscore the necessity for further research to fully understand the efficacy of antibiotics in preventing irinotecan-induced diarrhea and to refine treatment protocols accordingly.60
Atropine
Atropine serves as a competitive antagonist at muscarinic receptors, effectively blocking their activity.61 This action makes it particularly useful in preventing or mitigating the cholinergic side effects induced by irinotecan treatment, such as early-onset diarrhea. The recommended dosage for this purpose ranges from 0.25 to 1 mg, which can be administered either intravenously or subcutaneously.62-63 When employing atropine for these effects, it’s crucial to closely monitor the patient’s blood pressure and heart rate. Furthermore, adherence to dosage guidelines is essential; the British Columbia Cancer Agency advises that the cumulative dose of atropine should not exceed 1.2 mg. This precaution helps to manage the symptoms effectively while minimizing the risk of adverse reactions associated with atropine use.11
Budesonide
Budesonide, a potent synthetic corticosteroid, is available for administration both topically and orally. In the context of managing diarrhea, especially when there is a lack of response to loperamide, oral budesonide has shown promise. A recommended dose for such cases is 9 mg once daily, taken for a duration of three to five days. This approach aims to leverage the anti-inflammatory properties of budesonide to alleviate the symptoms of diarrhea.50 Furthermore, the efficacy of oral budesonide in preventing diarrhea associated with irinotecan chemotherapy has been evaluated through a rigorous scientific study. This research, conducted with a double-blind, placebo-controlled, randomized methodology, focused on the administration of oral budesonide at a dose of 3 mg three times a day (TID). The outcomes of this study highlighted a notable reduction in both the frequency and duration of diarrhea episodes among participants who received budesonide, compared to those who were given a placebo.51
Diphenoxylate
Diphenoxylate, a synthetic opiate derivative, functions similarly to loperamide by slowing intestinal motility, thereby aiding in the management of diarrhea.50-51 For the treatment of mild to moderate diarrhea (grades 1 and 2), a combination of diphenoxylate-atropine and loperamide may be employed. The typical dosage for diphenoxylate-atropine is 1 to 2 tablets taken every 6 to 8 hours, according to clinical needs and patient response.51
Despite its usage, the current body of medical literature does not provide substantial evidence to support the effectiveness of diphenoxylate-atropine as a standalone alternative to loperamide in treating chemotherapy-induced diarrhea (CID). This suggests that while diphenoxylate-atropine may be considered as part of a broader treatment strategy for CID, reliance on loperamide based on its established efficacy remains prevalent.52
Probiotic Management
Probiotics, which are live microorganisms known to confer health benefits upon their host, have garnered attention for their efficacy in treating various digestive conditions. These beneficial bacteria, including strains of lactobacilli, bifidobacteria, and streptococci, play a pivotal role in maintaining gut health. Imbalances in these microbial populations have been linked to a range of gastrointestinal disorders, such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), neonatal necrotizing enterocolitis, and Clostridium difficile colitis.64-65 Recent research underscores the significant therapeutic potential of probiotics, showcasing their anti-inflammatory effects in both animal models and human subjects with IBD. These findings suggest that the benefits of probiotics may extend beyond gastrointestinal health, potentially offering anticancer advantages due to their anti-inflammatory properties.66
In the context of oncology, probiotics have begun to be recognized as a viable treatment strategy for managing chemotherapy-induced diarrhea, particularly in patients undergoing treatment for colon cancer. Illustrative of their potential, a case study reported the successful use of probiotics in alleviating severe CID in breast cancer patients. This emerging evidence supports the broader application of probiotics in mitigating the adverse effects of chemotherapy on the digestive system, highlighting their importance in the integrative management of cancer treatment side effects.67
Nutritional Management
The Mediterranean Modified Healthy Diet (MMHD) is an innovative adaptation of the traditional Mediterranean diet, specifically designed by nutritionists to mitigate the risk of diarrhea, particularly for patients undergoing chemotherapy.68 This dietary plan incorporates the principles of the Mediterranean diet, known for its health benefits, including a high intake of fruits, vegetables, whole grains, and healthy fats, but with strategic modifications to address the specific nutritional needs and challenges faced by cancer patients. The MMHD aligns with the World Cancer Research Fund (WCRF) guidelines, emphasizing the importance of a healthy weight, regular physical activity, and a diet rich in whole grains, vegetables, fruits, and legumes, while minimizing the intake of fast food, red and processed meats, sugary drinks, and alcohol. Additionally, the WCRF advises against relying on dietary supplements for cancer prevention and promotes breastfeeding and adherence to their dietary recommendations post-cancer diagnosis. In the context of managing diarrhea, the MMHD takes a tailored approach by providing a carefully calculated daily caloric intake of 1818 kcal and a fiber intake of 22.90 to 30.36 g, aligning with or exceeding the minimum recommended daily fiber requirement.69 To minimize the risk of diarrhea, the diet restricts fiber intake by limiting the consumption of legumes, vegetables, fruits, and excluding whole grains. The variety and quantity of vegetables are also controlled to decrease stool frequency, and soft drinks are prohibited. Recognizing the prevalence of lactase non-persistence (LNP) and lactose intolerance, especially among Italians, lactose is omitted from the diet to further reduce the likelihood of digestive discomfort. The MMHD’s role in managing diarrhea for chemotherapy patients is significant. By carefully adjusting dietary fiber intake and excluding known dietary triggers such as lactose, this diet aims to provide optimal nutrition while minimizing the gastrointestinal side effects commonly associated with cancer treatment. This proactive nutritional strategy supports the overall well-being of patients during a challenging period, helping to maintain their quality of life and potentially enhancing the effectiveness of their cancer treatment.70-71
Herbal Management
The exploration of herbal management for chemotherapy-induced diarrhea (CID) presents an innovative approach, tapping into the wealth of traditional medicine and phytochemical research. Herbal formulations and plant extracts have been identified as promising candidates for mitigating the gastrointestinal (GI) toxicity associated with chemotherapeutic agents like CPT-11, leveraging the synergistic interactions of their chemical components to target various bodily processes simultaneously.72-73 One notable example is the Huangqin Decoction (HQD), a traditional Chinese medicine formula consisting of Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fisch, Paeonia lactiflora Pall, and Ziziphus jujuba Mill, mixed in a specific ratio. HQD has a long history, spanning over 1800 years, of being used to treat gastrointestinal disorders, including symptoms such as diarrhea, nausea, stomach cramps, and vomiting.74-75 Research indicates that HQD may influence the metabolism of lipids, bile acids, and certain amino acids, suggesting a complex mechanism of action beneficial for managing CID.76-77
Similarly, Hange-Shashin-to, a Japanese Kampo medicine composed of seven plants, has been traditionally used for treating acute gastroenteritis and diarrhea. The major flavonoid in Hange-Shashin-to, baicalin, has been shown in animal studies to reduce glucuronidase activity, which correlates with decreased weight loss, improved anorexia, and a delay in the onset of diarrhea symptoms. Additionally, Hange-Shashin-to is reported to inhibit glucuronidase activity, reduce the production of prostaglandin E2 in the gut, and enhance water absorption, offering a multifaceted approach to managing CID.78
Prevention of Cid
These examples underscore the potential of herbal medicines and phytochemicals in offering complementary strategies for the management and prevention of chemotherapy-induced diarrhea. By harnessing the natural synergies within herbal formulations, there is a promising avenue for developing multi-compound, multi-target therapies that could effectively reduce the GI toxicity associated with cancer treatment, highlighting the importance of integrating traditional herbal knowledge with modern clinical practices.79
Conclusion
In conclusion, the management and prevention of Chemotherapy-Induced Diarrhea (CID) represent critical components of supportive care in oncology. CID not only diminishes a patient’s quality of life but can also impede the effective administration of chemotherapy by necessitating dosage adjustments or treatment delays. This overview has highlighted the multifaceted strategies essential for addressing CID, including dietary modifications, the utilization of probiotics, and the implementation of targeted pharmacological interventions such as octreotide and herbal formulations. Dietary adjustments serve as a foundational approach, aiming to minimize gastrointestinal irritation and stabilize digestion through the consumption of bland, low-fat, and low-sugar foods. Probiotics emerge as a beneficial adjunct by promoting gut health and potentially reducing the incidence of CID through the maintenance of a balanced intestinal flora. Furthermore, pharmacological solutions like octreotide offer a means to directly mitigate the mechanisms underlying CID in patients at high risk or with a history of severe diarrhea during chemotherapy. Herbal management, drawing from traditional practices and contemporary research, introduces an innovative avenue for both treatment and prevention, highlighting the potential of plant-based compounds and extracts in reducing gastrointestinal toxicity and enhancing patient well-being. Collectively, these strategies underscore the importance of a proactive, comprehensive approach to managing CID. By integrating dietary, probiotic, pharmacological, and herbal interventions, healthcare providers can offer effective support to cancer patients, enabling them to complete their prescribed chemotherapy regimens with minimal interruption and improved quality of life. The ongoing research and development of new treatments and preventive measures will continue to enhance our ability to manage this challenging side effect, ultimately contributing to better outcomes for patients undergoing chemotherapy.
Acknowledgement
We would like to express our sincere gratitude to all the researchers and experts who have contributed to the field of “Managing Chemotherapy-Induced Diarrhea: Efficacy of Interventions for Cancer Patients.” Their work has been invaluable in providing the foundation for this review. Our heartfelt thanks go to our colleagues and mentors who have supported us in every step of this journey.
Conflict of Interest
None
Funding Sources
Not Applicable
References
- WHO: World Health Statistics 2019: Monitoring Health for the SDGs. Geneva, Switzerland, World Health Organization, 2018.
- Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006;6(8):603-612.
CrossRef - Nandakumar A, Gupta PC, Gangadharan P, et al. Geographic pathology revisited: Development of an atlas of cancer in India. Int J Cancer. 2005;s116:740–754.
CrossRef - Mathur P, Sathishkumar K, Chaturvedi M, et al. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol. 2020;6:1063-1075.
CrossRef - Sathishkumar K, Chaturvedi M, Das P, Stephen S, Mathur P. Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J Med Res. 2022;156(4&5):598-607.
- Wierda WG, Byrd JC, Abramson JS, et al. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 4.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(2):185-217.
- Ahmed AR, Hombal SM. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol. 1984;11(6):1115-1126.
CrossRef - Arnold RJG, Gabrail N, Raut M, Kim R, Sung JCY, Zhou Y. Clinical implications of chemotherapy-induced diarrhea in patients with cancer. The Journal of Supportive Oncology. 2005;3(3):227-232.
- Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. The Lancet Oncology. 2005;6(2):93-102.
CrossRef - Arbuckle RB, Huber SL, Zacker C. The Consequences of Diarrhea Occurring During Chemotherapy for Colorectal Cancer: A Retrospective Study. The Oncologist. 2000;5(3):250-259.
CrossRef - Kornblau S, Al B. Benson, Catalano R, et al. Management of Cancer Treatment–Related Diarrhea. Journal of Pain and Symptom Management. 2000;19(2):118-129.
CrossRef - Engelking C, Rutledge DN, Ippoliti C, Neumann J, Hogan CM. Cancer-related diarrhea: a neglected cause of cancer-related symptom distress. Oncology Nursing Forum. 1998;25(5):859-860.
- Cassidy J, Misset JL. Oxaliplatin-related side effects: Characteristics and management. Seminars in Oncology. 2002;29(5):11-20.
CrossRef - Malet-Martino M. Clinical Studies of Three Oral Prodrugs of 5-Fluorouracil (Capecitabine, UFT, S-1): A Review. The Oncologist. 2002;7(4):288-323.
CrossRef - Goldberg RM, Sargent DJ, Morton RF, et al. N9741: FOLFOX (oxaliplatin(Oxal)/ 5-fluorouracil (5-FU)/ leucovorin (LV) or reduced dose R-IFL (CPT-11 + 5-FU/LV) in advanced colorectal cancer (CRC): Final efficacy data from an intergroup study. Journal of Clinical Oncology. 2004;22(14):3621-3621.
CrossRef - Hospers G, Schaapveld M. Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid vs high dose 24h 5-FU infusion/folinic acid (FA) + oxaliplatin(OXA) in metastatic colorectal cancer (MCRC). Journal of Clinical Oncology. 2004;22(14):3539-3539.
CrossRef - André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343-2351.
CrossRef - de Gramont A, Figer A, Seymour M, et al. Leucovorin and Fluorouracil With or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. Journal of Clinical Oncology. 2000;18(16):2938-2947.
CrossRef - Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus Fluorouracil and Leucovorin for Metastatic Colorectal Cancer. New England Journal of Medicine. 2000;343(13):905-914.
CrossRef - Rothenberg ML, Meropol NJ, Poplin EA, Van Cutsem E, Wadler S. Mortality Associated With Irinotecan Plus Bolus Fluorouracil/Leucovorin: Summary Findings of an Independent Panel. Journal of Clinical Oncology. 2001;19(18):3801-3807.
CrossRef - Fordtran JS. Speculations on the pathogenesis of diarrhea. Fed Proc. 1967;26(5):1405-1414.
- Fasano A. Toxins and the gut: role in human disease. Gut. 2002;50 Suppl 3(Suppl 3):III9-III14.
CrossRef - Cash RA, Forrest JN, Nalin DR, Abrutyn E. Rapid correction of acidosis and dehydration of cholera with oral electrolyte and glucose solution. 1970;2:549–550.
CrossRef - Szilagyi A, Ishayek N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. 2018;10(12):1994.
CrossRef - Jordan K, Kellner O, Kegel T, Schmoll HJ, Grothey A. Phase II Trial of Capecitabine/Irinotecan and Capecitabine/Oxaliplatin in Advanced Gastrointestinal Cancers. Clinical Colorectal Cancer. 2004;4(1):46-50.
CrossRef - Davila M, Bresalier RS. Gastrointestinal complications of oncologic therapy. Nature Clinical Practice Gastroenterology & Hepatology. 2008;5(12):682-696.
CrossRef - Vincenzi B, Schiavon G, Pantano F, Santini D, Tonini G. Predictive factors for chemotherapy-related toxic effects in patients with colorectal cancer. Nature Clinical Practice Oncology. 2008;5(8):455-465.
CrossRef - Voigt W, Matsui S, Yin MB, Burhans WC, Minderman H, Rustum YM. Topoisomerase-I inhibitor SN-38 can induce DNA damage and chromosomal aberrations independent from DNA synthesis. Anticancer Research. 1998;18(5A):3499-3505. Accessed October 2, 2022.
- Gibson RJ, Stringer AM. Chemotherapy-induced diarrhoea. Current Opinion in Supportive and Palliative Care. 2009;3(1):31-35.
CrossRef - Saliba F, Hagipantelli R, Misset JL, et al. Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: a prospective assessment. Journal of Clinical Oncology. 1998;16(8):2745-2751.
CrossRef - Stringer AM, Gibson RJ, Logan RM, et al. Chemotherapy-induced diarrhea is associated with changes in the luminal environment in the DA rat. Experimental Biology and Medicine (Maywood, NJ). 2007;232(1):96-106. Accessed October 2, 2022.
- Takasuna K, Hagiwara T, Hirohashi M, et al. Inhibition of intestinal microflora β-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemotherapy and Pharmacology. 1998;42(4):280-286.
CrossRef - Fittkau M, Voigt W, Holzhausen HJ, Schmoll HJ. Saccharic acid 1.4-lactone protects against CPT-11-induced mucosa damage in rats. Journal of Cancer Research and Clinical Oncology. 2004;130(7):388-394.
CrossRef - Takasuna K, Kasai Y, Kitano Y, et al. [Study on the mechanisms of diarrhea induced by a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats]. Nihon Yakurigaku Zasshi Folia Pharmacologica Japonica. 1995;105(6):447-460.
CrossRef - Gibson RJ, Bowen JM, Inglis MRB, Cummins AG, Keefe DMK. Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. Journal of Gastroenterology and Hepatology. 2003;18(9):1095-1100.
CrossRef - Stringer AM, Gibson RJ, Logan RM, et al. Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemotherapy and Pharmacology. 2008;64(1):123-132.
CrossRef - Zalcberg J, Kerr D, Seymour L, Palmer M. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. European Journal of Cancer. 1998;34(12):1871-1875.
CrossRef - Benson AB, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2004;22(14):2918-2926.
CrossRef - Goumas, Naxakis, Christopoulou, Chrysanthopoulos, Nikolopoulou, Kalofonos. Octreotide Acetate in the Treatment of Fluorouracil-Induced Diarrhea. The Oncologist. 1998;3(1):50-53.
CrossRef - Gligorov J, Lotz JP. Preclinical pharmacology of the taxanes: implications of the differences. Oncologist. 2004;9 Suppl 2:3-8.
CrossRef - Eisenhauer EA, Vermorken JB. The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs. 1998;55(1):5-30.
CrossRef - Chen L, Fu W, Zheng L, Liu Z, Liang G. Recent Progress of Small-Molecule Epidermal Growth Factor Receptor (EGFR) Inhibitors against C797S Resistance in Non-Small-Cell Lung Cancer. J Med Chem. 2018;61(10):4290-4300.
CrossRef - Yang JC, Reguart N, Barinoff J, et al. Diarrhea associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther. 2013;13(6):729-736. doi:10.1586/era.13.31.
CrossRef - Loriot Y, Perlemuter G, Malka D, et al. Drug insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy [published correction appears in Nat Clin Pract Oncol. 2009 Mar;6(3):180. Penault-Lorca, Frédérique [corrected to Penault-Llorca, Frédérique]]. Nat Clin Pract Oncol. 2008;5(5):268-278.
CrossRef - Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol. 2010;2(1):51-63.
CrossRef - Benson AB, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2004;22(14):2918-2926.
CrossRef - Regnard C, Twycross R, Mihalyo M, Wilcock A. Loperamide. J Pain Symptom Manage. 2011;42(2):319-323.
CrossRef - Lenfers BH, Loeffler TM, Droege CM, Hausamen TU. Substantial activity of budesonide in patients with irinotecan (CPT-11) and 5-fluorouracil induced diarrhea and failure of loperamide treatment. Ann Oncol. 1999;10(10):1251-1253.
CrossRef - Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105-S120.
CrossRef - Gebbia V, Carreca I, Testa A, et al. Subcutaneous octreotide versus oral loperamide in the treatment of diarrhea following chemotherapy. Anticancer Drugs. 1993;4(4):443-445.
CrossRef - Barbounis V, Koumakis G, Vassilomanolakis M, Demiri M, Efremidis AP. Control of irinotecan-induced diarrhea by octreotide after loperamide failure. Support Care Cancer. 2001;9(4):258-260.
CrossRef - Zidan J, Haim N, Beny A, Stein M, Gez E, Kuten A. Octreotide in the treatment of severe chemotherapy-induced diarrhea. Ann Oncol. 2001;12(2):227-229.
CrossRef - Wadler S, Haynes H, Wiernik PH. Phase I trial of the somatostatin analog octreotide acetate in the treatment of fluoropyrimidine-induced diarrhea. J Clin Oncol. 1995;13(1):222-226.
CrossRef - Wasserman E, Hidalgo M, Hornedo J, Cortés-Funes H. Octreotide (SMS 201-995) for hematopoietic support-dependent high-dose chemotherapy (HSD-HDC)-related diarrhoea: dose finding study and evaluation of efficacy. Bone Marrow Transplant. 1997;20(9):711-714.
CrossRef - Glimelius B. Benefit-Risk Assessment of Irinotecan in Advanced Colorectal Cancer. Drug Safety. 2005;28(5):417-433.
CrossRef - Kehrer DF, Sparreboom A, Verweij J, et al. Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res. 2001;7(5):1136-1141.
- Schmittel A, Jahnke K, Thiel E, Keilholz U. Neomycin as secondary prophylaxis for irinotecan-induced diarrhea. Ann Oncol. 2004;15(8):1296.
CrossRef - de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin Pharmacokinet. 2018;57(10):1229-1254.
CrossRef - Flieger D, Klassert C, Hainke S, Keller R, Kleinschmidt R, Fischbach W. Phase II clinical trial for prevention of delayed diarrhea with cholestyramine/levofloxacin in the second-line treatment with irinotecan biweekly in patients with metastatic colorectal carcinoma. Oncology. 2007;72(1-2):10-16.
CrossRef - Yumuk PF, Aydin SZ, Dane F, et al. The absence of early diarrhea with atropine premedication during irinotecan therapy in metastatic colorectal patients. International Journal of Colorectal Disease. 2004;19(6):609-610.
CrossRef - Jansman FG, Sleijfer DT, de Graaf JC, Coenen JL, Brouwers JR. Management of chemotherapy-induced adverse effects in the treatment of colorectal cancer. Drug Safety. 2001;24(5):353-367.
CrossRef - Karthaus M, Ballo H, Abenhardt W, et al. Prospective, Double-Blind, Placebo-Controlled, Multicenter, Randomized Phase III Study with Orally Administered Budesonide for Prevention of Irinotecan (CPT-11)-Induced Diarrhea in Patients with Advanced Colorectal Cancer. Oncology. 2005;68(4-6):326-332.
CrossRef - Glimelius B. Benefit-Risk Assessment of Irinotecan in Advanced Colorectal Cancer. Drug Safety. 2005;28(5):417-433.
CrossRef - Balfour Sartor R, Muehlbauer M. Microbial host interactions in IBD: Implications for pathogenesis and therapy. Current Gastroenterology Reports. 2007;9(6):497-507.
CrossRef - El-Atti SA, Wasicek K, Mark S, Hegazi R. Use of Probiotics in the Management of Chemotherapy-Induced Diarrhea: A Case Study. Journal of Parenteral and Enteral Nutrition. 2009;33(5):569-570.
CrossRef - Artale S, Barzaghi S, Grillo N, et al. Role of Diet in the Management of Carcinoid Syndrome: Clinical Recommendations for Nutrition in Patients with Neuroendocrine Tumors. Nutrition and Cancer. 2020;1-10.
CrossRef - Castagnini C, Luceri C, Toti S, et al. Reduction of colonic inflammation in HLA-B27 transgenic rats by feeding Marie Ménard apples, rich in polyphenols. The British Journal of Nutrition. 2009;102(11):1620-1628.
CrossRef - Bayless TM, Rosensweig NS. A racial difference in incidence of lactase deficiency. A survey of milk intolerance and lactase deficiency in healthy adult males. JAMA. 1966;197(12):968-972.
CrossRef - Graf E. Chinese Drugs of Plant Origin. Chemistry, Pharmacology, and Use in Traditional and Modern Medicine. Von W. Tang und G. Eisenbrand. Springer-Verlag Berlin etc. 1992, X, 1056, S., 41 Abb. gebd. DM 248,00. Pharmazie in Unserer Zeit. 1992;21(6):281-281.
CrossRef - Wang Y, Fan X, Qu H, Gao X, Cheng Y. Strategies and Techniques for Multi-Component Drug Design from Medicinal Herbs and Traditional Chinese Medicine. Current Topics in Medicinal Chemistry. 2012;12(12):1356-1362.
CrossRef - Swami U, Goel S, Mani S. Therapeutic Targeting of CPT-11 Induced Diarrhea: A Case for Prophylaxis. Current Drug Targets. 2013;14(7):777-797.
CrossRef - Cui DN, Wang X, Chen JQ, et al. Quantitative Evaluation of the Compatibility Effects of Huangqin Decoction on the Treatment of Irinotecan-Induced Gastrointestinal Toxicity Using Untargeted Metabolomics. Front Pharmacol. 2017;8:211.
CrossRef - Kase Y, Hayakawa T, Aburada M, Komatsu Y, Kamataki T. Preventive effects of Hange-shashin-to on irinotecan hydrochloride-caused diarrhea and its relevance to the colonic prostaglandin E2 and water absorption in the rat. Japanese Journal of Pharmacology. 1997;75(4):407-413.
CrossRef - Kawashima K, Nomura A, Makino T, Saito K, Kano Y. Pharmacological properties of traditional medicine (XXIX): effect of Hange-shashin-to and the combinations of its herbal constituents on rat experimental colitis. Biological & Pharmaceutical Bulletin. 2004;27(10):1599-1603.
CrossRef - Narita M, Nagai E, Hagiwara H, Aburada M, Yokoi T, Kamataki T. Inhibition of beta-glucuronidase by natural glucuronides of kampo medicines using glucuronide of SN-38 (7-ethyl-10-hydroxycamptothecin) as a substrate. Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 1993;23(1):5-10.
CrossRef - Takasuna K, Kasai Y, Kitano Y, et al. Protective effects of kampo medicines and baicalin against intestinal toxicity of a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats. Japanese Journal of Cancer Research: Gann. 1995;86(10):978-984.
CrossRef - Yokoi T, Narita M, Nagai E, Hagiwara H, Aburada M, Kamataki T. Inhibition of UDP-glucuronosyltransferase by aglycons of natural glucuronides in kampo medicines using SN-38 as a substrate. Japanese Journal of Cancer Research: Gann. 1995;86(10):985-989.
CrossRef - Yamakawa JI, Motoo Y, Moriya J, et al. Significance of Kampo, traditional Japanese medicine, in supportive care of cancer patients. Evidence-Based Complementary and Alternative Medicine: eCAM. 2013;2013:746486.
CrossRef - Kornblau S, Benson AB, Catalano R, et al. Management of cancer treatment-related diarrhea. Issues and therapeutic strategies. J Pain Symptom Manage. 2000;19(2):118-129.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





