How to Cite | Publication History | PlumX Article Matrix
Leela Karunakaran* and Anita Rosemarie Joseph Singh
and Anita Rosemarie Joseph Singh
Department of Biotechnology, Women’s Christian College, Affiliated to the University of Madras, Chennai, Tamilnadu, India
Corresponding Author E-mail: k.leela1993@gmail.com
ABSTRACT: Plant parts contain diverse distribution of secondary compounds which plays a predominant role in promoting health and well-being along with vital roles in environmental and agricultural applications. In the present study, crude ethanol extracts prepared from Mangifera indica L., Piper betle L. and Lawsonia inermis L., leaves were subjected towards purification by column chromatography using hexane, chloroform, ethyl acetate and methanol in 4:3:2:1 ratio as column mobile phase. The obtained purified fractions were further resolved in thin layer chromatographic plates with hexane and ethyl acetate in 7.2:2.8 ratio as mobile phase. Best fraction from each of the three samples showing better separation was characterized by means of gas chromatographic – mass spectrometric analysis for determining the existence of valuable as well as pharmacologically active components. Totally 15 bioactive compounds were identified in each of these purified fractions wherein, 3 compounds were observed to be similar in the fractions of all three samples and includes Meso-2,3-Bis-T-Butoxysuccinonitrile; Methyl 3-Methyl-5-Oxy-2-Phenoxyhexanedithioate and 2,3,4,4-Tretrapropyl-1-(Trimethylsilyl)-1-(Trimethylsilyloxy)-1,3-Diaza-2,4-Diborabutane. Further investigation on these compounds could pave way towards development of drugs of therapeutic and industrial importance.
KEYWORDS: GC-MS - Gas Chromatography - Mass Spectrometry; Rf – Retention Factor; TLC – Thin Layer Chromatography; UV – Ultra Violet
| Copy the following to cite this article: Karunakaran L, Singh A. R. J. Purification and Identification of Secondary Compounds in Mangifera indica L., Piper betle L. and Lawsonia inermis L. Leaves by Column Chromatography and GC-MS Analysis. Biotech Res Asia 2025;22(1). |
| Copy the following to cite this URL: Karunakaran L, Singh A. R. J. Purification and Identification of Secondary Compounds in Mangifera indica L., Piper betle L. and Lawsonia inermis L. Leaves by Column Chromatography and GC-MS Analysis. Biotech Res Asia 2025;22(1). Available from: https://bit.ly/41DE8zm |
Introduction
Plants synthesize multifarious secondary compounds which are known to display potential roles in food industry, as therapeutics, in fragrances, cosmetics, environmental and medical applications.1 Based on their chemical nature, these phytocompounds are grouped into terpenes involving terpenoid compounds, polyphenolic compounds such as phenols, flavanoids, coumarins, lignins and tannins, sulphur containing compounds such as glucosinolates and nitrogen containing compounds such as alkaloids, cyanogenic glucosides and so.2-5 These compounds exert numerous pharmacological functions like antioxidant, anti-inflammatory, antimicrobial, anticancer, antidiabetic activity also, are utilized for treating hypertension, cardiovascular problems, arthritis, against several ailments thereby emphasizing the usage and effectiveness of different herbs in traditional medicine.6-8 Plant derived phytocomponents serve as a great source of drugs and since their nature is of much complexity there arises a necessity to isolate and purify these individual compounds to further analyze and assess their structural as well as functional properties.9 Thin layer chromatography and column chromatography are among such commonly used techniques which aid in the separation of active constituents from a crude mixture of plant extracts. It can be achieved through an optimal solvent system comprising either a single solvent or a mix of different solvents in varying ratios. Efficient migration of these compounds is also dependent on its nature, polarity and interaction with the stationary phase used. It can be visualized under light source or by spraying with chemical reagents.10 Gas chromatographic and mass spectrometric analysis is among the major and rapidly exploited technique which helps in identifying and quantifying the phytochemicals present in the plant extracts as well as in the purified fractions such as esters, alkaloids, fatty acids, volatile oils etc.11-14 Thus, numerous pharmacologically active compounds can be isolated from a single plant sample that could contribute towards an in depth understanding about the type of constituents present as well as their utility and beneficial importance towards multitudinous applications.
In this study, ethanol crude extracts from Mangifera indica L., Piper betle L. and Lawsonia inermis L., leaves were purified by column chromatography using an optimized solvent system containing hexane, chloroform, ethyl acetate and methanol in a specific ratio as the mobile phase and the fractions thus obtained were resolved in TLC and suitable fractions from all three samples were analyzed by gas chromatographic and mass spectrometric technique for determining the bioactive compounds present in these fractions.
Materials and Methods
Sample collection, authentication and extraction
Mangifera indica L., Piper betle L. and Lawsonia inermis L. leaf samples was procured from places in and around Chennai and authenticated by Prof Jayaraman, Director at Plant Anatomy Research Centre, Chennai with authentication numbers PARC/2021/4406, PARC/2021/4405 and PARC/2021/4407. Dried and powdered plant leaf samples were extracted using ethanol in the ratio of 1:10 nd has been reported in our previous paper.15,16 Crude ethanol extracts obtained from all three plant leaf samples were subjected to TLC profiling and column chromatography for isolation and purification of bioactive constituents.
TLC profiling of crude ethanol extracts
Ethanol crude extracts from Mangifera indica L., Piper betle L. and Lawsonia inermis L. were profiled by Thin Layer Chromatography with varying mobile phase of differing ratios and has already been performed and published in our earlier study.16 Among the studied solvent systems, hexane, chloroform, ethyl acetate and methanol in 4:3:2:1 ratio showed efficient separation and migration of compounds. The bands were visualized under visible and UV light and derivatized in iodine chamber. Based on TLC profiling studies, the optimized solvent system [hexane, chloroform, ethyl acetate and methanol in 4:3:2:1] was chosen as mobile phase for column chromatographic purification.
Column chromatographic purification of ethanol crude extracts
Crude ethanol extracts from Mangifera indica L., Piper betle L. and Lawsonia inermis L. was loaded onto the glass column with stationary phase comprising silica gel of 100 – 200 mesh and mobile phase comprising hexane, chloroform, ethyl acetate and methanol in 4:3:2:1 ratio. The column purified fractions collected from all three plant samples were individually run in TLC plate with hexane and ethyl acetate (7.2 : 2.8) as mobile phase. Similar fractions were pooled together and labelled as Mangifera indica L. fraction, Piper betle L. fraction and Lawsonia inermis L. Fraction. Bands were derivatized under UV light, iodine chamber, anisaldehyde sulphuric acid reagent and methanolic sulphuric acid reagent.17 Rf (Retention factor) value of the spots or bands were calculated using the formula18:
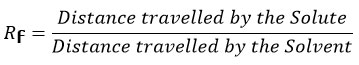
Gas chromatography – mass spectrometric analysis
About 1-2 mL of the column purified fractions from Mangifera indica L., Piper betle L. and Lawsonia inermis L. were determined by Gas chromatographic and mass spectrometric analysis using Shimadzu QP2020 instrument with SH-RXi-5Sil MS column and helium as the flow gas with operating conditions of 50°C column oven temperature, 250°C injection temperature, 68.1 kPa pressure, 16.2 mL per min and 1.20 mL per min as total and column flow, 39.7 cm per sec as linear velocity, purge flow of 3.0 mL per min, 200°C and 250°C as ion source and interface temperature. Temperature of 50°C for 0 mins and 280°C for 2 mins as oven temperature program. Component spectrums were matched with database containing spectrums of well-known compounds stored in the GC-MS NIST and WILEY library
Data analysis and Presentation
Rf values of the derivatized bands or spots in TLC were analyzed by calculating the distance travelled by the solute (purified fraction) and solvent (hexane and ethyl acetate in ratio of 7.2: 2.8). The obtained Rf value was observed to be similar for all derivatizations. GC-MS identification of bioactive constituents in the purified fractions were carried out by comparing their spectrums with known compounds in NIST and WILEY library. The analytical data corresponding to the same are given in Figures 1-2 and Tables 1-4.
Results
About 1-2 grams of crude ethanol extracts rom Mangifera indica L., Piper betle L. and Lawsonia inermis L., leaves were individually loaded on to the glass column with stationary phase containing 100-200 mesh size of silica gel and the compounds were eluted using mobile phase comprising hexane, chloroform, ethyl acetate and methanol in 4:3:2:1 ratio for purification of bioactive constituents by means of column chromatography. Totally, 25 purified fractions were obtained from Mangifera indica L., 28 purified fractions from Piper betle L. and 31 purified fractions from Lawsonia inermis L., The collected purified fractions were further run in TLC plate with mobile phase containing hexane and ethyl acetate in 7.2:2.8 ratio for compound resolution. Fractions showing similar migration and separation of bands with better resolution were combined together as a single fraction in all three samples (Figure 1) and derivatized under UV light, iodine chamber, anisaldehyde sulphuric acid reagent and methanolic sulphuric acid reagent (Figure 2). These purified active fractions from all three plant leaf samples were determined for the presence of phytocompounds by means of gas chromatographic and mass spectrometric analytical method. Formation of colored bands by derivatization showcased the existence of innumerable secondary components in purified fractions. Rf values of the fraction from all three samples are given (Table 1).
 |
Figure 1: Column purified fractions from Mangifera indica L., Piper betle L. and Lawsonia inermis L. |
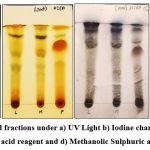 |
Figure 2: Derivatized fractions under a) UV Light b) Iodine chamber c) Anisaldehyde Sulphuric acid reagent and d) Methanolic Sulphuric acid reagent. |
Table 1: Rf values of the purified fractions
| S. No | Fractions | Rf values [For all derivatizations] |
| 1. | Lawsonia inermis L. Fraction | 0.4, 0.54, 0.68, 0.74, 0.82, 0.94 |
| 2. | Mangifera indica L. Fraction | 0.54, 0.68, 0.82, 0.97 |
| 3. | Piper betle L. Fraction | 0.48, 0.68, 0.80, 0.94 |
Column purified fractions were analyzed by GC-MS technique to assess the nature of phytocompounds present. GC-MS analysis of column purified fractions from Mangifera indica L., Piper betle L. and Lawsonia inermis L. showed the presence of about 15 bioactive compounds in each of the sample fractions and are tabulated (Table 2 – 4).
Table 2: GC-MS report for compounds from Mangifera indica L.
| Peak | Retention time | Compounds |
| 1 | 13.868 | 2,3,4,4-Tretrapropyl-1-(Trimethylsilyl)-1-(Trimethylsilyloxy)-1,3-Diaza-2,4-Diborabutane |
| 2 | 17.476 | 1,3-Diphenyl-1-((Trimethylsilyl)oxy)-1(Z)-Heptene |
| 3 | 20.712 | Tri-O-Trimethylsilyl, N-Pentafluoropropionyl derivative of Terbutaline |
| 4 | 25.959 | Methyl 6 – Hydroxycaproate |
| 5 | 32.901 | Meso-2,3-Bis-T-Butoxysuccinonitrile |
| 6 | 34.764 | 1,2-Benzenedicarboxylic acid, Dioctyl ester |
| 7 | 37.371 | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester |
| 8 | 38.621 | Methyl 3-Methyl-5-Oxy-2-Phenoxyhexanedithioate |
| 9 | 38.72 | Ethyl Cis-4-Methyl-3-((E)-2-Phenylethenyl) Cyclohepta-1,5-Diene- 1-Carboxylate |
| 10 | 38.782 | 6-Bromo-5-Oxo-3-Methylhexan-1,3-Diol |
| 11 | 38.9 | 2-Fluoro-5-trifluoromethylbenzoic acid, propyl ester |
| 12 | 38.925 | 2-Fluoro-5-trifluoromethylbenzoic acid, undecyl ester |
| 13 | 38.955 | 2-Fluoro-5-trifluoromethylbenzoic acid, pentyl ester |
| 14 | 39.017 | 6-Bromo-5-Oxo-3-Methylhexan-1,3-Diol |
| 15 | 39.065 | Carbonic acid, 2 – Bromo-3,3- Diphenyl -2-Propenyl ethyl ester |
Table 3: GC-MS report for compounds from Piper betle L.
| Peak | Retention time | Compounds |
| 1 | 13.866 | 2,3,4,4-Tretrapropyl-1-(Trimethylsilyl)-1-(Trimethylsilyloxy)-1,3-Diaza-2,4-Diborabutane |
| 2 | 32.89 | Meso-2,3-Bis-T-Butoxysuccinonitrile |
| 3 | 34.761 | 1,2-Benzenedicarboxylic acid, Dioctyl ester |
| 4 | 36.137 | 2,5-Dimethyl-1,3,5-Dithiazine |
| 5 | 37.373 | 1,3-Butadien-1-ol, acetate |
| 6 | 39.164 | Methyl 3-Methyl-5-Oxy-2-Phenoxyhexanedithioate |
| 7 | 39.23 | Benzofuro [3,2-B] Pyridine-1(2H)-Carboxylic acid, 3-Cyano-4-Methyl-, Ethyl ester |
| 8 | 39.389 | Ethyl (1R*, 2S*, 11R*) – (+-) -3,10-Dioxo-2,11-Epoxycyclodecane-1-carboxylate |
| 9 | 39.46 | 5-Azido1-Phenyl-3-Methyl-4- [2-Azido-2- (Ethoxy carbonyl) Ethenyl] Pyrazole |
| 10 | 39.505 | Ethanediamide, N, N’-Bis [2-[[(3,5-Dimethoxyphenyl) Amino] Oxo acetyl] Phenyl]- |
| 11 | 39.605 | Butanedioic acid, 2-Ethyl-2-(Phenylthio)-, 1-Ethyl ester |
| 12 | 39.63 | 2-(3-Oxo-2-Pent-2-Enyl-Cyclopentyl)-Acetamide |
| 13 | 40.105 | Methyl 3-Methyl-5-Oxy-2-Phenoxyhexanedithioate |
| 14 | 40.17 | N-(T-Butyl)-2-Benzoylbenzamide |
| 15 | 40.205 | Arenarol |
Table 4: GC-MS report for compounds from Lawsonia inermis L.
| Peak | Retention time | Compounds |
| 1 | 13.873 | 2,3,4,4-Tretrapropyl-1-(Trimethylsilyl)-1-(Trimethylsilyloxy)-1,3-Diaza-2,4-Diborabutane |
| 2 | 17.481 | 1,3-Diphenyl-1-((Trimethylsilyl)oxy)-1(Z)-Heptene |
| 3 | 20.715 | Phosphonous Dibromide, [2,2,2-Trifluoro-1-(Trifluoromethyl)-1-[(Trimethylsilyl)oxy] Ethyl]- |
| 4 | 25.961 | Methyl 6 – Hydroxycaproate |
| 5 | 29.14 | Methyl 6 – Hydroxycaproate |
| 6 | 32.892 | Meso-2,3-Bis-T-Butoxysuccinonitrile |
| 7 | 34.764 | 1,2-Benzenedicarboxylic, Dicyclohexyl ester |
| 8 | 39.165 | 2-Fluoro-3-trifluoromethylbenzoic acid, 6- Chlorohexyl ester |
| 9 | 39.23 | 2-Fluoro-3-trifluoromethylbenzoic acid, octyl ester |
| 10 | 39.416 | Propanoic acid, 2-Methyl-, 5-(2,3-Dihydro-3,3-Dimethyl-2-Oxo-1H-indol-1-yl)-10,11-dihydro-5H-Dibenzo [A, D] Cyclohepten-5-yl ester |
| 11 | 39.664 | Methyl 3-Methyl-5-Oxy-2-Phenoxyhexanedithioate |
| 12 | 39.831 | 1,3-Dioxane-4, 6-Dione, 5- [(Cyclohexyl methylamino) Methylene] -2,2-Dimethyl- |
| 13 | 39.915 | Benzo [F] [1,7] Naphthyridine – 2- Carboxylic acid, 5,6- Dihydro – 3- Methyl -5 – Oxo -, Ethyl ester |
| 14 | 40.031 | 2-Fluoro-5-triflouromethyl benzoic acid, pentyl ester |
| 15 | 40.22 | Ethyl (1R*, 2S*, 11R*) – (+-) -3,10-Dioxo-2,11-Epoxycyclodecane-1-carboxylate |
Discussion
Derivatization helps in enhancing the visualization of secondary compounds since, these reagents act by interacting with the analyte giving off coloured derivatives that enables easier and rapid detection of components.19 Derivatization of active fractions from Mangifera indica L., Piper betle L. and Lawsonia inermis L. under UV light, iodine chamber, anisaldehyde sulphuric acid reagent and methanolic Sulphuric acid reagent has resulted in Rf values of 0.4, 0.54, 0.68, 0.74, 0.82, 0.94 in L. inermis L., 0.48, 0.68, 0.80, 0.94 in P. betle L. and 0.54, 0.68, 0.82, 0.97 in M. indica L. Certain compounds were found to be present in all three samples such as Meso-2,3-Bis-T-Butoxysuccinonitrile; 2,3,4,4-Tretrapropyl-1- (Trimethylsilyl)-1-(Trimethylsilyloxy)-1,3-Diaza-2,4-Diborabutane and Methyl 3-Methyl-5-Oxy-2-Phenoxyhexanedithioate. Susanti et al.,20 has reported mobile phase comprising hexane, chloroform, ethyl acetate and rmic acid in 20:70:9:1 ratio for TLC separation of hexane extracts of Garcinia cowa Roxb bark. Jadhav and Ghatage21 has reported the use of hexane : ethyl acetate (50:50) as mobile phase for TLC separation of methanolic leaf extracts of Asplenium indicum with anisaldehyde and iodine reagent. Samrot AV et al.,22 has reported TLC bioautography studies for acetone, ethanol, petroleum ether and chloroform extracts of Mangifera indica L. leaves using chloroform and methanol in the ratio of 9:1 as mobile phase, derivatized under iodine and displayed Rf values of 0.09, 0.16, 0.20, 0.25, 0.98 for ethanol extract, 0.07, 0.20, 0.27, 0.98 for acetone extract, 0.05, 0.58, 0.78, 0.99 for chloroform extract and 0.04, 0.08, 0.14, 0.22, 0.97 for petroleum ether extract. Valle, Demetrio Jr et al.,23 has reported TLC bioautography and GC-MS studies on Philippine Piper betle L. leaf ethanol extracts using ethyl acetate and hexane in the ratio of 7:3 as mobile phase, derivatized with vanillin-sulfuric acid, visualized under visible light, UV 254 nm and UV 366 nm and were found to have displayed Rf values of 0.013, 0.13, 0.25, 0.40, 0.53, 0.70, 0.76, 0.86 and 0.92 in all derivatizations. GC-MS analysis of ethanol extracts have reported the presence of about 6 compounds such as Ethyl diazoacetate, Eugenol, Heptafluorobutyrate, 3-Fluoro-2-propynenitrite, 4-(2-Propenyl)phenol and Tris(trifluoromethyl)phosphine respectively. Kidanemariam T. K et al.,24 has reported the identification of bioactive constituents in hexane extract and essential oil of Lawsonia inermis L. leaves by GC-MS analysis and structural elucidation by NMR studies. Hexane extracts have been purified by column chromatography with hexane and ethyl acetate solvent system in the ratio of 7:3 and characterized by NMR technique which revealed the presence of Bisabolene compound in hexane extract while, GC-MS analysis of essential oil has displayed the presence of compounds such as hexadecanoic acid, eugenol, α-terpineol, phytol and ether phenyl vinyl etc. Ghosh K and Bhattacharya TK25 has reported column chromatographic isolation and GC-MS analysis of compounds from alcoholic and petroleum ether extract of Piper betle L. roots using eluate comprising petroleum ether and benzene and has led to identification of aristololactam A-II characterized to be 4-allyl resorcinol in alcohol extract and stigmast-4-en-3,6-dione, diketosteroid in petroleum ether extract. Dennis Amaechi et al.,26 has reported GC-MS analysis of ethanol extracts from Mangifera indica L. leaves which revealed the presence of compounds such as Eicosyl propyl ether, Dodecanoic acid, 2-Hexyne, 2-Methyl-E,E-3,13-octadecadien-1-ol, 9,12-Octadecadienoic acid, methyl ester, (E, E)-, Oleic acid, 1,14-Tetradecanediol, 1-(p-Toluidino)-1-deoxy-.beta.-d-Idopyranose, Morpholine, 4-methyl-, 4-oxide, Eicosyl propyl ether etc. whereas, in this study, ethanol extracts from Mangifera indica L., Piper betle L. and Lawsonia inermis L. were individually purified by column chromatography using a fixed mobile phase comprising hexane, chloroform, ethyl acetate and methanol in 4:3:2:1 ratio. Collected fractions were further run in hexane, ethyl acetate solvent system in 7.2:2.8 ratio and the best fraction showing active separation was characterized by GC-MS analysis and this led to the identification about 15 bioactive constituents from each of the sample fractions. Thus, TLC based separation along with purification of natural products by column chromatography gives us an insight about the nature and distribution of phytochemicals thereby rendering a possibility of deriving novel compounds with functional properties for multifarious approaches.
Conclusion
In this study, crude ethanol extracts from Mangifera indica L., Piper betle L. and Lawsonia inermis L. leaves were subjected to column chromatographic purification and isolation. Suitable fractions from all three samples were determined for the presence of bioactive constituents by GC-MS analysis. Purification studies were carried out employing a specific mobile phase combination of hexane, chloroform, ethyl acetate and methanol in 4:3:2:1 ratio. Further studies can be carried out on exploring and explicating the pharmacological and environmental utility of these compounds in various sectors.
Acknowledgement
We would like to acknowledge Heber Analytical Instrumentation Facility (HAIF), Bishop Heber College, Trichy for providing GC-MS analytical facility.
Funding Source
This work has been catalyzed and financially supported by Tamilnadu State Council for Science and Technology, Dept of Higher Education, Government of Tamilnadu through RFRS (Research funding for Research Scholars) Scheme TNSCST/RFRS/VR/09/2018-2019/7357
Conflict of Interest
The authors do not have any conflict of interest
Data availability Statement
This statement does not apply to this article
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Clinical Trial Registration
This research does not involve any clinical trials
Permission to reproduce material from other sources
Not Applicable
Author Contributions
Leela.K – Conducted experiment, Data Collection and analysis, Preparation of Manuscript
Anita R J Singh – Supervised the work, Reviewed and Finalized the Manuscript.
References
- Bolouri P, Salami R, Kouhi S, et al. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules. 2022; 27(24): 8999. DOI: https://doi.org/10.3390/molecules27248999.
CrossRef - Lyubenova, A., Georgieva, L., and Antonova, V. Utilization of plant secondary metabolites for plant protection. Biotechnology & Biotechnological Equipment. 2023; 37(1). DOI: https://doi.org/10.1080/13102818.2023.2297533.
CrossRef - Zaynab M, Fatima M, Abbas S, et al. Role of secondary metabolites in plant defense against pathogens. Microb Pathog. 2018; 124: 198–202. DOI: 10.1016/j.micpath.2018.08.034.
CrossRef - Ahmed, E., Arshad, M.N, Khan, M.Z, et al. Secondary metabolites and their multidimensional prospective in plant life. J Pharmacognosy Phytochem. 2017; 6(2): 205–214.
- Wink M. Evolution of secondary metabolites in legumes (Fabaceae). S Afr J Bot. 2013; 89: 164–175. DOI: 10.1016/j.sajb.2013.06.006.
CrossRef - Khade, O., Sruthi K., Sonkar, R., Gade, P., and Bhatt, P. Plant secondary metabolites: Extraction, screening, analysis and their bioactivity. International Journal of Herbal Medicine. 2023; 11(2): 01-17. DOI: https://doi.org/10.22271/flora.2023.v11.i2a.855.
CrossRef - Koche, D., Shirsat, R., and Kawale, M.A. An Overview of Major Classes of Phytochemicals: Their Types and Role in Disease Prevention. Hislopia Journal. 2016; 9: 01-11.
- Alaekwe, I., Ajiwe, V., and Aguoma, C. Phytochemical Screening and Chromatographic Purification of Bauhunia semibifida ROXB Hexane-Dichloromethane Leaf Extract. Journal of Materials Science and Chemical Engineering. 2023; 11: 14-19. DOI: 4236/msce.2023.117002.
CrossRef - Kumar, V. Phytochemical Screening and Thin Layer Chromatography Profiling of Various Extracts of Achyranthes aspera and Cissus quadrangularis. The Journal of Phytopharmacology. 2021; 10(4): 225-229. DOI: 10.31254/phyto.2021.10402.
CrossRef - Ahamed, T., Rahman, S.M., and Shohael, A.M. Thin layer chromatographic profiling and phytochemical screening of six medicinal plants in Bangladesh. International Journal of Biosciences. 2017; 11(1): 131-140. DOI: 10.12692/ijb/11.1.131-140.
CrossRef - Thamer, FH and Thamer, N. Gas chromatography – Mass spectrometry (GC-MS) profiling reveals newly described bioactive compounds in Citrullus colocynthis (L.) seeds oil extracts. Heliyon. 2023; 9(6): e16861. DOI: https://doi.org/10.1016/j.heliyon.2023.e16861.
CrossRef - El-Naggar, H.M., Shehata, A.M., and Morsi, MA.A. Micropropagation and GC–MS analysis of bioactive compounds in bulbs and callus of white squill. In Vitro Cell. Biol.-Plant. 2023; 59: 154–166. DOI: https://doi.org/10.1007/s11627-023-10333-9.
CrossRef - Srivastava, R., Mukerjee, A., and Verma, A. GC-MS Analysis of phytocomponents in pet ether fraction of Wrightiatinctoria Pharmacogn J. 2015; 7: 249–253. DOI: https://doi.org/10.5530/pj.2015.4.7.
CrossRef - Saravanakumar, K., Raj, A., and Umaiyambigai, D. GC-MS and FT-IR profiling of leaves methanol extract from the Pleiospermium alatum (Wall. ex Wt. & Arn) Swingle Rutaceae family. J Phytopharm. 2016; 5(5): 201–204. DOI: https://doi.org/10.31254/phyto.2016.5506.
CrossRef - Leela K and Singh R. J. Phytochemical Screening and Antibacterial Activity of Three Medicinal Plants – Lawsonia inermis l., Mangifera indica l. and Piper betel l. Biosc. Biotech. res. Comm. 2020; 13 (2): 848-859. DOI: https://doi.org/10.21786/bbrc/13.2/70.
CrossRef - Leela K and Singh A. R. J. Tlc profiling and GC-MS Analysis of Secondary metabolites From Medicinal Plants – lawsonia inermis l., Mangifera indica l. & Piper betle l. International Journal of Recent Scientific Research. 2022; 13(08): 1979-1983. DOI: https://doi.org/10.24327/ijrsr.2022.1308.0415.
- Bidkar A, Sable V, and Kirtane S. Extraction, characterization and pharmacological activity and evaluation of various extracts of calotropis procera leaves. International Journal of Health Sciences. 2021; 5(S2): 1182–1204. https://doi.org/10.53730/ijhs.v5nS2.14413
CrossRef - Lalrinzuali K, Vabeiryureilai M and Ganesh Chandra J. Phytochemical and TLC Profiling of Oroxylum indicum and Milletia pachycarpa. J Plant Biochem Physiol. 2015; 3:152.
CrossRef - Alfa Chemistry. Enhancing Thin Layer Chromatography (TLC) Capabilities with New Derivatization Reagents. Lab insights. https://labinsights.nl/en/article/enhancing-thin-layer-chromatography-tlc-capabilities-with-new-derivatization-reagents. 29 April, 2024. 15 January, 2025.
- Susanti M, Pratama AR, Suryani MI, Suryati, D. Development and validation of TLC-densitometry method for quantification of tetraprenyltoluquinone in the stem bark hexane extract of Garcinia cowa Heliyon. 2022; 8(9): e10437. DOI: 10.1016/j.heliyon.2022.e10437.
CrossRef - Jadhav, D and Ghatage, M. Isolation and Characterization of Secondary Metabolites of Asplenium indicum by Using Thin Layer Chromatography. Journal of Scientific Research. 2021; 65(6): 137-141. DOI: 10.37398/JSR.2021.650623.
CrossRef - Samrot AV, Rohan B, Kumar D, Sahiti K, Raji P and Samanvitha KS. Detection of antioxidant and antibacterial activity of Mangifera indica using TLC bio-autography. Int J Pharm Sci Res 2016; 7(11): 4467-72. DOI: 10.13040/IJPSR.0975-8232.7(11).4467-72.
CrossRef - Valle D Jr, Puzon JJ, Cabrera E and Rivera W. Thin Layer Chromatography-Bioautography and Gas Chromatography-Mass Spectrometry of Antimicrobial Leaf Extracts from Philippine Piper betle against Multidrug-Resistant Bacteria. Evidence-Based Complementary and Alternative Medicine. 2016; 1-7. 10.1155/2016/4976791.
CrossRef - Kidanemariam TK, Tesema TK, Asressu KH, Boru AD. Chemical Investigation of Lawsonia inermis Leaves from Afar Region, Ethiopia . Orient J Chem. 2013; 29(3). doi : http://dx.doi.org/10.13005/ojc/290339
CrossRef - Ghosh K and Bhattacharya TK. Chemical constituents of Piper betle (Piperaceae) roots. Molecules. 2005; 10(7), 798–802. https://doi.org/10.3390/10070798
CrossRef - Dennis A, Ini P E, Benjamin N Y and Theresa F A. Phytochemical Profiling (GC-MS) of the Leaf Extract of Mangifera indica and its Hypolipidemic Effect on Serum Lipid Profile in Streptozotocin-Induced Diabetic Wistar Rats. Asian Sci. Bull. 2024; 2(1): 17-23. DOI: https://doi.org/10.3923/asb.2024.17.23
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





