How to Cite | Publication History | PlumX Article Matrix
B. Boboye* and I. Dayo-Owoyemi
Department of Microbiology, Federal University of Technology, P. M. B. 704, Akure, Ondo State (Nigeria)
ABSTRACT: Effects of raw and boiled extracts of garlic on some human pathogenic bacteria: Vibrio cholerae, Proteus mirabilis, Salmonella paratyphi B and C, Shigella dysenteriae, Pseudomonas aeruginosa and Staphylococcus aureus were examined by agar diffusion method. Minimum inhibitory concentration (MIC) of crude garlic extract was determined using P. aeruginosa and S. aureus by tube dilution method. It was found that raw garlic extract inhibited the growth of all the microbes to varying degrees at 24 hr of incubation. Action of the extract appeared bacteriostatic on V. cholerae, P. mirabilis, S. paratyphi B and C and S. aureus. At 48 hr of incubation, it was observed that P. aeruginosa and S. dysenteriae did not resume growth in the presence of the extract. None of the pathogens was prevented from growing in the presence of boiled garlic extract. The minimum inhibitory concentrations of the crude garlic extract were 134 mg/ml and 161 mg/ml for P. aeruginosa and S. aureus respectively.
KEYWORDS: Antibacterial efficacy; Minimum inhibitory concentration; Allium sativum; human pathogens
Download this article as:| Copy the following to cite this article: Boboye B, Dayo-Owoyemi I. Antibacterial Effect and Minimum inhibitory concentrations of Garlic (Allium sativum) extracts on some human pathogens. Biosci Biotechnol Res Asia 2003;2(1) |
| Copy the following to cite this URL: Boboye B, Dayo-Owoyemi I. Antibacterial Effect and Minimum inhibitory concentrations of Garlic (Allium sativum) extracts on some human pathogens. Biosci Biotechnol Res Asia 2003;2(1). Available from: https://www.biotech-asia.org/?p=3420 |
Introduction
Garlic (Allium sativum) is commonly used as a spice and in the treatment of some diseases. Some of the traditional uses of garlic include the cure of cold, flu, infectious ear aches, vaginal yeast infections and high blood pressure¹. Modern researches have focused on the application of garlic in the treatment of heart diseases, cancer and antioxidant effect2,3,1,4. Certain studies have also shown that the plant possesses antimicrobial property. A long time before microbes were discovered, French priests of the middle ages were using garlic against bubonic plague now known to be a bacterial infection4.
In the late 19th century, research studies on effect of the garlic plant on infections have intensified5,1,6. Antibacterial, antifungal and antiviral actions of garlic have been reported by Hughes and Lawson7. Garlic extract was reported to be active against the growth of Escherichia coli, Pseudomonas aeruginosa, Aspergillus flavus, Bacillus cereus, Corynebacterium diphtheriae, Candida albicans, Staphylococcus aureus, Streptococcus faecalis, Salmonella typhimurium and influenza virus8,9,10,11,4,12. In a study carried out by Boboye et al.12, it was reported that the extracts of garlic, onion (Allium cepa) and ginger (Zingiber officinale) were effective against the growth of Klebsiella pneumoniae amongst other bacteria tested. Garlic extract prevented the growth of Trichophyton rubrum and Microsporium auduini11. Garlic has antiparasitic action. Nork et al.14 reported that garlic pulp completely suppressed the ability of Trypanosma brucei to cause African trypanosmiasis in mice.
In order to assess the ability of garlic extract to cure diseases caused by some commonly encountered bacteria in Nigeria, a survey of the effect of the spice on V. cholerae, P. mirabilis, S. paratyphi B and C, S. dysenteriae, P. aeruginosa and S. aureus was investigated. Minimum inhibitory concentrations were determined for P. aeruginosa and S. aureus.
Materials and Methods
Sources of Materials
Fresh bulbs of garlic were obtained from “Oba” market in Akure, Ondo State, Nigeria.
cholerae, mirabilis, S. paratyphi B and C, S. dysenteriae, P. aeruginosa and S. aureus were provided by Federal Medical Centre, Owo, Ondo State and Institute of Medical Research, Yaba, Lagos State, Nigeria.
Preparation of Extracts
blender was surface-sterilized by using absolute ethanol for 30 min, drained and allowed to dry. Raw extract was made by grinding 100 g of the garlic in 150 ml sterile distilled water. This was boiled for 20 min to obtain boiled extract. Crude extract was prepared by filtering the raw extract through a sterile sieve with pore size of 0.1 mm.
Agar Diffusion Test
An aliquot (0.5 ml) of 18 hr old nutrient broth culture (OD600, 0.45) of each of the bacteria was spread on nutrient agar. An hole was bored into the agar with 14 mm cork borer and filled with the raw extract. Control plate contained sterile distilled water instead of the extract. Another control plate was prepared as the test plate but lacked the organism substituted with sterile nutrient broth. All plates were incubated at 37oC for 24 to 48 hr and observed for clear zone which indicates sensitivity of the test organisms to the garlic. Degree of sensitivity was determined by measuring the diameter in the zone of inhibition in millimetre. This procedure was carried out three times and repeated using boiled garlic extract.
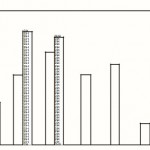 |
Figure 1: Effect of raw garlic extract on some human pathogenic bacteria Inhibition to extract at 24 hr of incubation: Inhibition to extract at 48 hr of incubation |
Determination of Minimum Inhibitory Concentration
This test was carried out by tube dilution method with crude garlic extract using P. aureginosa and S. aureus selected to represent Gram positive and Gram negative bacteria. A 0.5 ml of 18 hr P. aeruginosa (20 x 107 cells) and S. aureus (26 x 107 cells) were added to 9.5 ml peptone water contained in test tube. Crude extract was added to obtain varying final concentrations of 0.067, 0.134, 0.201, 0.268, 0.335, 0.402, 0.469 and 0.536 g/ml. Two sets of control tubes were prepared. A set contained nutrient broth substituted for the bacterium. Another set of control tubes lacked the extract replaced with distilled water. All tubes were incubated at 37oC for 24 hr. Transparency at the top of the liquid in the tube was recorded as inhibition. Aliquot (0.1 ml) of this liquid was inoculated into nutrient agar in order to confirm death of the bacterial cells. Garlic concentration in tube corresponding to where complete death of the organism was observed first was noted as the point where minimum inhibitory concentration started.
The above described procedure used for minimum inhibitory concentration (MIC) test was repeated to determine the specific MIC for each of the bacteria with final extract concentrations of 0.067, 0.0804, 0.094, 0.107, 0.121 and 0.134 g/ml for P. aeruginosa and 0.134, 0.147, 0.161, 0.174, 0.188 and 0.201 g/ml for S. aureus.
Results and Discussion
At 24 hr of incubation, S. aureus appeared to be the most sensitive microbe to the raw garlic extract with the largest diameter (19 mm) in the zone of inhibition. This was followed by that of S. dysenteriae (17.3 mm), V. cholerae (15mm), S. paratyphi C (13.5 mm), P. mirabilis (13 mm), P. aeruginosa (13 mm) and S. paratyphi B which showed the least sensitivity with 4 mm diameter of growth inhibition (Fig. 1). At 48 hr of incubation, diameters of inhibition for all but two (P. aeruginosa and S. dysenteriae) of the microbes decreased to 8, 0, 0, 0 and 0 mm respectively. The zones of inhibition for the P. aeruginosa increased to 21 and that of dysenteriae to 20 mm. Zones of inhibition were completely covered with cells of S. paratyphi B and C, P. mirabilis and V. cholerae. This shows a resumption of full growth of these organisms.
Boiled garlic extract did not prevent the growth of any of the bacteria. On the other hand in the tube dilution MIC test, P. aeruginosa and S. aureus appeared susceptible to crude garlic extract starting from 134 mg/ml and 201 mg/ml. Data obtained from further MIC study showed that the minimum inhibitory concentrations for the two organisms were 134 mg/ml and 161 mg/ml respectively.
The findings in this work reveal that the raw garlic extract had inhibitory effects on the tested organisms. Resumed growth of some of the microbes observed at 48 hr of incubation suggests that the garlic has a bacteriostatic action on the bacteria. Garlic effect on P. aeruginosa and S. dysenteriae appeared bacteriocidal. In contrast, boiled garlic was ineffective against all the bacteria. In a clinical study, it was shown that cooking destroys the ability of garlic to produce allicin4. The result obtained in this work therefore suggests that the active constituent/s in the extract has/have been deformed, evaporated or destroyed by the applied heat. Principal ingredients in garlic are sulphur containing volatile oils which include allicine, ajoene, allyl sulphides and vinyldithins15,4.
The MIC of the P. aeruginosa and S. aureus were close to each other with the former being most susceptible. These concentrations were higher than the standard value (30 mg/ml) recommended for MIC and those of many antibiotics such as gentamycin, tetracycline, erythromycin, ampicillin, tobramycin, kanamycin and chloramphenicol commonly used against these two organisms16. This could be attributed to the reason that the garlic is a plant containing other consitituents while the conventional antibiotics are purified substances synthesized by microorganisms wholly or partially from chemical compound which at low concentrations inhibit the growth of other microbes17. However, in a study carried out by Singh and Shukla18, it was reported that garlic was more effective against clinical strains of Staphylococcus, Escherichia, Proteus, Pseudomonas and Klebsiella than the antibiotics (penicillin, ampicillin, deoxycycline, streptomycin and cephalexin) that they tested. Thus, based on the results obtained in this study and the fact that garlic is non toxic to human when consumed orally, garlic extract can be used to cure infectious diseases which are caused by the P. aeruginosa, S. aureus and similar microbial agents sensitive to the action of the plant. It is recommended that the extract should be purified and further studied.
Acknowledgement
We are grateful to Mr F. Akharaiyi for technical assistance and Department of Microbiology, Federal University of Technology, Akure, Ondo State, Nigeria for instrumentation and provision of materials.
References
- Bergner, P., The Healing Power of Garlic, Prima Publishing Coy. (1996)
- Stephen, F. and Blackwood, J., Garlic: Nature is Original Remedy Healing, Arts Press. 1-4 (1991)
- Warshafsky,, Kamar, R. and Sivak, S., Effect of Garlic on total serum cholestrol: A meta-analysis. Ann. Int. Medica, 17 (7), 599-605 (1993)
- Koch, H. P. and Lawson, L. D., Garlic. The Science and Therapeutic Application of Allium sativum and related Spices, 2nd Williams and Wilkins, Baltimore, 62-64 (1996)
- Adetumbi, M. and Lau, B. H., Allium sativum (Garlic) : A natural antibiotic, Medical Hypothesis, 12, 227-237 (1983)
- Newall, C. A., Anderson, L. A. and Phillipson, J. D., Garlic in Herbal Medicine. A guide for health care professionals, Pharmaceutical Press, London, 129-133 (1996)
- Hughes, B. G. and Lawson, L. D., Antimicrobial effect of Allium sativum (Garlic), Allium ampeloprasum (Elephant garlic) and Allium cepa (Onion), garlic compounds and commercial garlic supplement products. Res., 5, 154-158 (1991)
- Desrosier, N. W. and Desrosier, T. N., The Technology of Food Preservation. 4th edition. AVI Publ. INC. West Port Connecticut, 558 (1977)
- Shelef, L. A., Antimicrobial effects of spices. Journal of Food Safety, 6, 29-44 (1983)
- Rajabather, K., Chiropractic and Nutrition. Designer food for the health of it. Townsend letter for Doctors, 1170-1172 (1994)
- Venugopal, P. V. and Venugopal, T. V., Antidermatophytic action of Allium sativum (Garlic) in-vitro. International Journal of Dermatology, 34 (4), 278-279 (1995)
- Boboye B., Babatunde T. and Onoriode A., Antibacterial Activities of Some Plants used as Condiments and Spices in Nigeria. Journal of Technoscience, Submitted (2003)
- De, N., Bassey, M. and Sale, M., Antibacterial spectrum of extracts of Zingiber officinale (Ginger) and Allium sativum (Garlic). 25th Annual Conference of Nigerian Society of Microbiology, 10 (2001)
- Nork, A. J., Williams, S. and Onyenekwe, P. C., Allium sativum induced death of African trypanosomes. Parasitology Research, 82, 634-637 (1996)
- Dutta, A. C., Botany for Degree Students. 2nd Oxford University Press, London, 704 (1968)
- Brock, T. D., Smith, D. W. and Madigan, M., Biology of Microorganisms. 4th Prentice-Hall International Inc., U. S. A., 516 (1994)
- Pelczar, M. J., Chan, E. C. and Kreig, N. R., Microbiology: Concept and Application. McGraw-Hill International, 578, 683, 694 (1989)
- Singh, D. V. and Shukla, N. P., Activity of multiple resistant bacterial of garlic extract. Fitoterapia, 5, 313-315 (1984)

This work is licensed under a Creative Commons Attribution 4.0 International License.





