How to Cite | Publication History | PlumX Article Matrix
Contamination by Polycyclic Aromatic Hydrocarbons in Some Egyptian Mediterranean Coasts
Y. M. Moustafa
Egyptian Petroleum Research Institute, Nasr City, Cairo (Egypt)
ABSTRACT: The distribution of individual PAHs of the reference 16 PAHs listed by the United States Environmental Protection Agency (US-EPA) using high performance liquid chromatography (HPLC) was determined in tar ball, water and sediment samples. Eight sites with a total of 24 samples were chosen for this study. The sources of PAHs contamination (pyrogenic and petrogenic) were achieved using both PAHs distribution and molecular indices based on ratios of selected PAH concentrations. The contamination is shown to be either petrogenic or mixed petrogenic and pyrolytic. Levels of total PAHs were found to vary between 8.36μg/g and 438.38μg/g for tar ball samples. Water and sediment samples have values ranging between (8.00 ppm -12.08 ppm) and (3.75-4.35 μg/g dry weight.) respectively. The obtained values can be considered to possess serious health risk to both human health and all living organisms.
KEYWORDS: Contamination; aromatic hydrocarbons; Egyptian mediterranean coasts
Download this article as:| Copy the following to cite this article: Moustafa Y. M. Contamination by Polycyclic Aromatic Hydrocarbons in Some Egyptian Mediterranean Coasts. Biosci Biotechnol Res Asia 2003;2(1) |
| Copy the following to cite this URL: Moustafa Y. M. Contamination by Polycyclic Aromatic Hydrocarbons in Some Egyptian Mediterranean Coasts. Biosci Biotechnol Res Asia 2003;2(1). Available from: https://www.biotech-asia.org/?p=3414 |
Introduction
Oil pollution problems have become the focus of increasing regulatory, public and research concern because of its adverse negative impacts on human health and the environment (El-Tokhi and Moustafa, 2001). The group of chemical compounds known as polycyclic aromatic hydrocarbons (PAHs) represents an important class of compounds that affect to the environment. They are a class of organic pollutants ubiquitous in the aquatic ecosystem, showing lipophilic character and resistant to biodegradation. (Kirs et al., 1986). They have toxic, mutagenic and /or carcinogenic properties. They are also highly lipid-soluble and thus readily absorbed from the gastrointestinal tract of mammals (Samanta et al.2002, King et al., 2004).
Investigation of (PAHs) in the aquatic environment is a very important part of environmental quality assessment that determines the status of contamination and the likely impacts it may cause to the ecosystem (Maskaoui, et al., 2002). The PAHs contamination may result from either pyrogenic sources (incomplete combustion of organic matter, emission sources and exhausts) or from the release of petroleum into the environment (Parhal and Carpenter, 1983). After entering the environment, PAHs are widely dispersed by atmospheric transport or through stream pathways, and eventually accumulate in soil and aquatic sediments. The microorganisms (naturally occurring or genetically engineered) can mineralize toxic PAHs into CO2 and H2O (Samanta, et al., 2002).
There is lack a of information in the levels and the distribution of PAHs in the Egyptian environment. Accordingly the purpose of the present work is to identify the levels and the distribution of individual PAHs in order to establish an up to date PAHs environmental monitoring useful as a reference for future studies and conservation programs.
Material and Methods
Sampling
Eight sites were chosen for this study with a total of 24 samples (tar ball, water and sediment) The names and locations of these sites is shown in Table (1). Surface water samples were collected using narrow neck borosilicate glass bottles with teflon lined caps. The samples were acidified to pH 2 using 10% HCl to preserve them against bacterial action during transportation and storage. Surface Sediment (0-2 cm) samples were collected using a stainless steel grab. Sediments from individual stations were well mixed and stored at refrigerator in pre-cleaned jars until analysis (Readman et al., 2002).
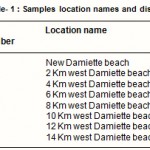 |
Table 1 : Samples location names and distance |
Oil Extraction
Extraction of oil from tar ball samples was carried out using soxhlet-extraction.20 g of tar ball samples were Soxhlet extracted using chloroform, the extraction was continued till the chloroform becomes colorless (Zakaria et al., 2001) 10 g of the dry homogenized sediment were placed in a soxhlet-extraction thimble mixed with 10 g of sodium sulfate. Extraction was carried out with methylene chloride/acetone mixture 1: 1 v/v for 24 hour (Viguri et al., 2002)
Oil was extracted from water samples using carbon tetrachloride (CCl4) as the method described by (Moustafa et al.,1997). The extract was dried in a sodium sulfate column and then cleaned up through with silica-gel column in order to remove the polar components.
Aromatic Extraction
The asphaltene fraction was extracted first from tar ball samples by precipitation with n-pentane as the method described briefly by Barakat et al., 1999.Then the aromatic fraction was separated using liquid column chromatography packed with activated neutral alumina (activated by heating at 300 °C for 24 hour). Myhre et al., 1990, describe the method.
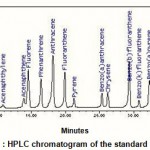 |
Figure 1 : HPLC chromatogram of the standard PAHs. |
Polycyclic Aromatic Hydrocarbon Analysis
PAH identification and quantification were performed using HPLC.The apparatus used was model Waters HPLC 600E, equipped with dual UV absorbance detector Waters 2487 and auto sampler Waters 717 plus attached to computerized system with Millennium 3.2 software. PAHs standards were obtained from Supelco. The condition of separation (Lal B. and Khanna S., 1996) is as follow:
Column:Supelcosil. LC-PAH, 5µm particles, 15cm length and4.6mm ID,
Mobile phase: Gradient acetonitrile: water 60 to 100 % acetonitrile (v/v) over 45 minute.
Flow rate: 0-2 min. 0.2 ml/min., 2-45 min. 1.0 ml/min.
Detector: Set at 254 nm.
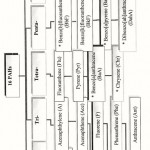 |
Figure 2: Nomenclatures of 16 PAHs standard according to number of rings and their abbreviations. (*) are carcinogenic compounds. |
Results and Discussion
The distribution of individual PAHs of the reference 16 PAHs listed by the United States Environmental Protection Agency (USEPA) (Ke, et al., 2002) using HPLC is shown in Fig. -1. These PAHs can be divided according to the number of rings into low and high molecular weights PAHs.The low molecular weights consist of two and three aromatic rings which are 6 LPAHs.While the high molecular weight consists of tetra-, penta- and hexa-aromatic rings, they are 10 HPAHs (Viguri, et al., 2002). The nomenclatures and abbreviations of the 16 PAHs standard according to number of rings are shown in Fig. – 2.
Tar Ball Samples
Qualitative identification of the individual PAHs for the extracted oils from tar ball samples is shown in Figs – 3 and 4. Careful examination of the HPLC chromatograms shows that, each station has its own distribution pattern and all have most of target compounds. The brief identification of the present PAHs as shown in Table – 2 reveal the presence of both LPAH and HPAH.
Low molecular weight PAHs cause acute toxicity, whereas some of the higher molecular weight PAHs is carcinogenic. The toxicity occurs when UV excites the electrons in PAHs, resulting in the formation of toxic singlet oxygen as a by-product. The toxic singlet oxygen can damage biological membranes (Hatch and Burton,1999).
PAH distribution is the most useful tool in distinguishing pyrogenic from petrogenic origins of PAHs contamination. All samples as shown in Table-3 are characterized by the predominance of HPAH over the LPAH. It has been recognized that in general, pyrogenic PAHs are characterized by the dominance of the high HPAHs over the LPAHs.In contrast petrogenic PAHs are normally abundant in lower molecular weight compounds (LPAHs), which are readily modified by weathering or degradation (Wang et al., 1999). Thus all sites were contaminated by either pyrogenic or degraded petrogenic origin of PAHs. The concentrations of total PAHs (the sum of 16 PAHs) are ranging between 8.36-and 438.38 µg/g, as reported in Table-2. Highest concentrations were observed at site 3.The presence of such high concentrations are considered to be toxic. All sites are characterized by relatively high concentrations of four membered ring compared to the 2-, 3-, 5- and 6-membered rings.
A less subjective approach to investigate sources can be achieved using molecular indices based on ratios of selected PAHs concentrations (Readman et al., 2002). They were chosen according to their thermodynamic stability. Among the three ring isomers phenanthrene is thermodynamically more stable than anthracene.and among the four-ring isomers fluoranthene is thermodynamically less stable than pyrene. Petrgenic favors the formation of thermodynamically more stable PAHs, while pyrolysis at high temperature generates the less stable isomers. Phenanthrene/anthracene (Phe/Ant) and fluoranthene/pyrene (Flu/Pyr) have been used to distinguish between pyrolytic and petrogenic origin of PAHs Phe/Ant ratios higher than 10 are seen in petroleum inputs and values lower than 10 are characteristic of pyrogenic source (Tolosa I. et al., 2004). Regarding to Phe/Ant ratio, sites No.4 and 5 have values > 15 indicating petrogenic origin of the PAHs, while sites No.1 and 6 have values < 10 indicating pyrogenic origin. Sites No. 2, 3, 7 and 8 have values of infinity due to the absence of anthracene indicating purely petrogenic origin. Flu/ Pyr ratio < 1 are characteristic of petrogenic origin and >1for pyrogenic origin. Results from the fluoranthene/pyrene ratio show that all samples except sample No 4, have low values <1 indicating petrogenic origin of PAHs whereas sample No. 4 has value more than unity indicating pyrogenic origin of PAHs.
In conclusion, there appear some discrepancies between the results obtained using the different parameters. Weathering causes considerable changes in the chemical and physical properties of spilled oils as well as the PAHs (Zakaria, et al., 2001). Due to evaporation, dissolution and biodegradation, low molecular weights PAHs are thought to be selectively disappeared. As weathering increases the summation of the 6 LPAHs decreased which makes the Ó 6 LPAHs: Ó 10 HPAHs parameter unuseful to detect the origin. On the other hand it could be useful in the comparison of the weathering effects on the samples (Zakaria, et al., 2001). Data obtained show lower ratios indicating that all the tar ball samples had undergone weathering in different degrees. Sites can be arranged according to their ascending degree of weathering as follows: 8, 2 and 7,4,5 and 6, 1and 3. Based on that weathering show pronounced decrease in naphthalene relative to the other PAHs.All stations, except station 3 show the absence of naphthalene, confirming that all stations suffered from weathering. Also station 3 with its detected value (48.76 µg/g) may be considered as the least affected by weathering. Thus PAHs composition could be used as one of the indices of weathering.
 |
Figure 3: HPLC chromatograms of the PAHs extracted from tar balls, sites 1,2,3 and 4 |
 |
Figure 4: HPLC chromatograms of the PAHs extracted from tar balls, sites 5,6,7 and 8 |
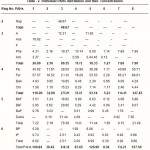 |
Table 2 :Individual PAHs distribution and their concentrations |
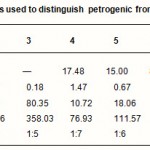 |
Table 3: PAHs parameters used to distinguish petrogenic from pyrogenic origin of PAHs |
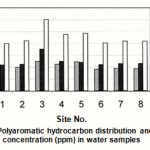 |
Figure 5 : Polyaromatic hydrocarbon distribution and their concentration (ppm) in water samples |
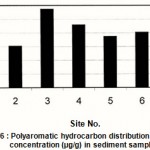 |
Figure 6 : Polyaromatic hydrocarbon distribution and their concentration (µg/g) in sediment samples |
Water Samples
HPLC analysis of all the extracted oils shows the same PAHs pattern Only phenanthrene and benzo(a)pyreneare detected but with different concentratios.Their concentrations ranges are (3.58 ppm -5.02 ppm) and (4.42 ppm -7.06ppm) for phenanthrene and benzo(a)pyrene are respectively as shown in the histogram Fig. 5. The total PAHs concentrations are shown to be between 8.00 and 12.08 ppm. The highest value is observed at site 3. The absence of the other PAHs may be due to the highly hydrophobic nature of these compounds and hence their strong interactions with suspended particles, leads to their removal from the water column. In addition, other important processes such as volatilization, photodegradation and biological mediation may remove a proportion of these compounds (Maskaoui et al., 2002).
Phenanthrene is known to be a photosensiter of human skin, a mild allergen and mutagenic to bacterial systems under specific conditions. However, the toxicity of benzo(a)pyrene has been studied and there is suffient experimental evidence to show that it is carcinogenic (Samanta et al., 2002).
Sediment Samples
The distribution profiles of PAHs for all the extracted oils are shown to have the same profile, Benzo(a)pyrene (5-membered ring) is the only compound detected. The concentrations are ranging between 3.75 and 4.35 µg/g dry weight as shown in histogram Fig. 6. The highest value is observed at site3 while the lowest value at site 6. The presence of such compound may have detrimental effect on the flora and fauna resulting in the uptake and accumulation in food chain (Harman et al., 2004)
The absence of the other PAHs may come to the fact that PAHs in sediments resulting from the release of petroleum were thought to be readily available for microbial degradation under aerobic conditions.
References
- Barakat M.A-K,Shimy Th.M. and Moustafa Y.M. Mineralogy of asphaltenes separated from crude oils and tar pollutants. Petroleum Science and Technology, 17(7&8).677-691 (1999)
- El-Tokhi M.M. and Moustafa Y.M. Heavy metals and petroleum hydrocarbons Contamination of bottom sediment of El-Sukhna area, Gulf of Suez, Egypt. Petroleum Science and Technology, 19(5,6), 481-494 (2001)
- Hartmann P.C.;Quinn J.G.;Cairns R.W. and King J.W. The distribution and sources of polycyclic aromatic hydrocarbons in Narragansett Bay surface sediments. Marine Pollution Bulletin, 48(3-4), 351-358 (2004)
- Hatch A.C. and Burton G.A. Photo-induced toxicity of PAHs to Hyalella azteca and chironomus tentans: effect of mixtures and behavior. Environmental Pollution,106, 157-167 (1999)
- Ke L.; Teresa, W.Y. Wong; Wong Y. S., and Nora, F. Y. Tam. Fate of polycyclic aromatic hydrocarbon (PAH) contamination in a mangrove swamp in Hong Kong following an oil spill. Marine Pollution Bulletin. 45, 339-347 (2002)
- King A.J.;Readman J.W. and Zhou J.L. Dynamic behavior of polycyclic aromatic hydrocarbons in Brighton marina,U.K., Marine Pollution Bulletin 48(3-4) 229-239 (2004)
- Kirso U.; Paalme L.; Kullik, M. and Irha, N. Monitoring of carcinogenic hydrocarbons in the Baltic Sea. Baltic Sea Environmental Proceedings, 19, 479-487.
- Lal B. and Khanna S. (1996): Degradation of crude oil by Acinetobacter calcoaceticus and Alcaligenes odorans, J. Appl. Bacterol., 81, 355-362 (1986)
- Maskaui K. ; Zhou J.L. ; Hong H. S. and Zhang Z.L. Contamination by polycyclic aromatic hydrocarbons in the Jiulong River estuary and western Xiamen Sea, China. Environmental Pollution, 118, 109-122 (2002)
- Moustafa Y.M.; Abdallh R.I. and Abd El Naby I.M. Levels of Hydrocarbons and Source Identification of Pollutants Contaminating Ismailya Canal. Journal of the Faculty of Education, 22,705-716 (1997)
- Myhre M.B.;Schon L.;Skjete T. and Karre J. Separation of maltenes into saturates, aromatics and O,N,S compounds. Geochem. 16(3-6), 931-941 (1990)
- Parhal, F.J. and Carpenter R. Polycyclic aromatic hydrocarbons (PAH)- Phase associations in Washington coastal sediment. Cosmochim. acta, 47, 1013-1023 (1983)
- Readman J.W.; Fillmann G.; Tolosa I.; Bartocci J.; Villeneuve I-P; Catinni C. and Mee L.D. Petroleum and PAH contamination of the Black Sea. Marine Pollution Bulletin, 44, 48-62 (2002)
- Riedel R.; Schlenk D.; Frank D.; and Costa-Pierce B., Analyses of organic and inorganic contaminations in Salton sea fish. Marine Pollution Bulletin, 44, 403-411 (2002)
- Samanta S.; Singh O.V.; and Jain R.K., Polycyclic aromatic hydrocarbons :environmental pollution and bioremediation. TRENDS in Biotechnology, 20, 243-248 (2002)
- Tolosa I.,Mora S., Sheikholeslami M.,Villeneuve J., Bartocci J and Cattini C.,Aliphatic and aromatic hydrocarbons in coastal Caspain Sea sediments. Marine Pollution Bulletin, 48 (1-2), 44-60 (2004)
- Viguri J.; Verde J., and Irabien A. Environmental assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Sanander Bay, Northern Spain. Chemosphere, 48, 157-165 (2002)
- Wang Z.; Fingas M.and David P. Oil spill identification. Journal of Chromatography A, 843, 369-411 (1999)
- Zakaria, M.P.; Okuda, T.; and Takada H. Polycyclic aromatic hydrocarbons (PAHs) and hopanes in stranded tar- balls on the Coastal of Peninsular Malaysia, Application of biomarkers for identifying sources of oil Pollution.Marine Pollution Bulletin, 42, (12), 1357-1366 (2001)

This work is licensed under a Creative Commons Attribution 4.0 International License.





