How to Cite | Publication History | PlumX Article Matrix
Cascade Operation of Stirred Tank Reactors for Alkaline Protease Production by Bacillus subtilis
Annapurna Jetty*, Ch. Subhakar, K. Kranthi And Ravichandra P.
Bioengineering and Environmental Centre, Indian Institute of Chemical Technology (CSIR), Tarnaka, Hyderabad - 500 007 India
ABSTRACT: The present work describes and compares stirred tank reactor (STR) with bioreactor (STR) in cascade of 4 and 6 for protease production using Bacillus subtilis. This gives an insight into the physiological state/ performance of the reactor based on product formation. The specific activity of protease showed an increasing trend from shake flask to STR and cascade reactors of 4 & 6 series. In shake flask the maximum specific activity was recorded during 72 hours with 7.339 Units/ ml, whereas in STR an increased enzyme activity of 10.106 Units/ml. has been achieved during the same period. The increase in enzyme activity is 52% from shake flask to STR. Similarly when cascade of STR (4 & 6 series) were used the maximum specific activity in cascade of 4 series of reactor is 13.71 Units/ml and 14.1 Units/ml in 6 series of reactor. The maximum activity achieved in gradostat is 38% more when compared to STR. The high performance of the cascade reactors are explained keeping in view of the physiological state of addition of external initial inoculum/ biomass, which is different from STR. The increase in enzyme production might be due to physiological state of the organism with respect to its origin. The production achieved in 4th bioreactor onwards is at steady state and the organism metabolic activity remains same irrespective of number of bioreactors in the series.
KEYWORDS: Bioreactors; Bacillus subtilis; Gradostat; Protease; STR
| Copy the following to cite this article: Jetty A, Subhakar C, Kranthi K, Ravichandra P.Cascade Operation of Stirred Tank Reactors for Alkaline Protease Production by Bacillus subtilis. Biosci Biotech Res Asia 2006;3(2a). |
| Copy the following to cite this URL: Jetty A, Subhakar C, Kranthi K, Ravichandra P.Cascade Operation of Stirred Tank Reactors for Alkaline Protease Production by Bacillus subtilis. Biosci Biotech Res Asia 2006;3(2a). Available from: https://bit.ly/3hdMqFR |
Introduction
Enzymes are efficient and precise catalysts that can be used in a variety of industrial and agricultural processes. Enzymatic processes have considerable advantages. Enzymes have an enormous potential to contribute to the development of cleaner, more effective, less energy consuming and completely new production process.¹ The most important industrial enzymes proteases account for nearly 60% total enzyme sales with 2/3 of protease being microbial origin². Additionally, proteolytic enzymes have been used for a long time in various industrial applications and different forms of therapy.2-6 Their use in medicine is notable, based on several clinical studies indicating their benefits in oncology, inflammatory conditions, blood rheology control and immune regulation.7,8
The discovery of new highly specific proteases and improved enzyme technology such as immobilization and novel reactor systems made the microbial systems even more attractive in biotechnology9,10. At present, about 60% of the commercially available enzymes are produced by Bacillus species, mostly being homologous proteins that are naturally secreted in the growth medium11,12. A bi-directional compound chemostat using a system of interconnecting vessels called the Gradostat was developed independently by Lovitt and Wimpenny in the year 1981 to model opposing solute gradients using small laboratory fermentation vessels.13,14 The use of Gradostat in production of microbial enzymes was described by Fredrickson,15 where two or more cascade of STR’s are connected in series, which have inoculum introduced into them from the previous reactor.
New sophisticated bioreactor designs with unique performance will play a vital role in the economic manufacture of useful biotechnological products from natural and genitically modified cell systems of microbial, mammalian and plant origin. The use of non-mechanically agitated bioreactors such as ALR and FBR may contribute to reducing the process cost and increase the production.16 The present study describes improved production of protease in cascade reactors of series four and six of Frederickson model, where an external source of biomass is introduced in every reactor unlike batch, fed-batch, and single stage STR. The study is also extended to see whether this increase in enzyme production is correlated with the increase in the number of Bioreactors and the same is compared with the STR.
Materials And Methods
Microorganism
Microorganism used for the production of protease enzyme in the present study was Bacillus subtilis. The organism was grown on growth media (nutrient agar, Hi media). The parent culture was subcultured every month to maintain fresh stock of the organism. The production media used in the bioreactor was a modified medium17. The production media used in the bioreactor contained, g/1L: Casein 10.0, Malt extract 10.0, Polypeptone 10.0, Sodium carbonate 10.0, Distilled water, pH 9.5.
Enzyme Assay
Tyrosine standard graph and protein standard graph were prepared using Casein and BSA as standards to estimate the specific enzyme produced in the bio reactors18,19. The specific activity of protease is defined as the amount of Tyrosine in milli moles per minute per mg of protein (Units/ml).
Shake Flask Method
50 ml of production media was prepared and taken in clean 250 ml conical flask and sterilized in autoclave at 15 lbs for 20 min. This was inoculated with 5% for 24 hr. old culture of B. subtilis and was kept at room temperature on a shaker at 150rpm for 4 days. Samples were drawn every 24hrs and simultaneously protein and tyrosine estimation were conducted to know the specific activity of the enzyme.
Design and operation of STR – 600 ml of production media was prepared and poured into the reactor bottle, which is fitted with air inlet and air outlet. A sample collection tube was fitted in the second port, which is dipped into the medium. The whole apparatus was sterilized at 15 lbs for 20 min. 24 hour old culture broth of Bacillus subtilis was used for inoculation. The reactor was fitted with air filters (0.2 µ) to allow only sterile air to pass and also air outlet. A magnetic bead was kept inside the reactor for agitating the media. During agitation to prevent the foam formation antifoaming agent (silicon oil) was used. Samples were drawn from the reactor for four days. Stirring speed of 250 rpm and constant air flow rate of 1 vvm (maintained by Rotameter) were used. Tyrosine content and protein content were estimated. From these values specific activity was calculated and expressed in terms of Units/ml.
Design and Operation of Bioreactors
In the preparation of cascade bioreactors, 500ml saline bottles were used. Silicon tubes were fitted to the bottles with air pumps. In the experiment, a series of 4 and 6 bottles were attached. Approximately 1 vvm of air was passed into the bottles using rotameter, for aeration and agitation. All the reactors were filled with 300ml of sterile production media. The first bottle was inoculated with 5% of 24hr. old inoculum. Subsequently the same amount of inoculum was transferred every 24 hours to the following bottles. Every day samples were collected for the enzyme and protein assays. These reactors were operated at 28±5°C.
Results And Discussion
Initially the production of protease has been studied in shake flask in the above said production media with maximum protease activity recorded during 72 hr period at 150 rpm at 7.34 Units/ml. Maximum amount of enzyme units were recorded in STR also during the same period at 10.106 Units/ml.
 |
Figure 1: Production of Protease in Shake flask and Stirred tank reactor |
Fig. -1 shows specific enzyme production of Shake flask and STR during the course of fermentation from 24 hr. to 72 hr. after inoculation. From the above graph it is evident that maximum enzyme production has been achieved during 72hours both in shake flask (7.339 units/ml) and STR (10.106 units/ml). A higher yield of enzyme in stirred tank reactor is well established by many earlier workers15,20 as explained by good aeration and agitation when compared with shake flask.
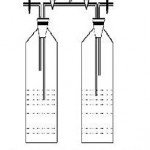 |
Figure 2: Schematic diagram of cascade of 2 bioreactors with air inlet and air out let and samples ports |
Fig. -2 shows the schematic diagram of cascade of two bioreactors with air inlet, air out let and sample ports. In the present experiment 4 and 6 series of bioreactors were used. A rotameter was connected to the air pump and the air flow rate was kept constant.
 |
Figure 3: Protein and Protease activity in four series of bioreactors |
Fig. -3 shows the protein and protease production in 4 series of bioreactors. From the results it is evident that maximum enzyme production in terms of specific activity of 13.71 Units/ ml is recorded in 4th bioreactor during 72 hours. The protein and specific activity has almost remained constant in 3rd and 4th bioreactors with 6.92-6.32 mg/ml of protein and 12-13.7 Units/ml of enzyme activity. The maximum enzyme activity is recorded in 4th reactor with 13.71 Units/ml. This shows the gradual increase in enzyme production from 9.2 to 13.7 Units/ml.
 |
Figure 4: Specific activity of protease in six series of bioreactors |
To further assess the enzyme production, and to see whether we can still increase the production as we increase the number of reactors, 6 series of reactors have been set up. Figure 4 shows interesting results, as there is not much increase beyond 4th bioreactor, where the maximum specific activity is around 13 Units/ml as comparable with 4 series experiment. In the 5th and 6th bioreactors the enzyme production remained almost at a steady state at 13.8 – 14 units/ml of specific enzyme activity.
Considering the various stages of a cascade of STR’s, the population in the first stage is homogenous, in the sense that its population is a collection of clones of its initial biomass. The second stage of population is not homogenous, because its population is mixture of clones of its initial biomass and of clones of initial biomass of first stage. Moreover because biomass is continuously being transferred from the first stage to the second and the chemical environments in different reactors are different, clones of initial biomass of the first stage that are in second stage have different past histories of environmental circumstances since their ancestral cells passed from first to the second stage at different times. This will be true when both the reactors are well mixed, and when steady states have been achieved in both. The population in the third stage will be even more inhomogeneous with respect to two factors of ultimate origin and history of environmental conditions than that in the second stage and that this trend is increasing, heterogeneity will continue to increase with stage number in the cascade13,21.
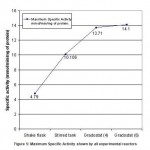 |
Figure 5: Maximum Specific Activity shown by all experimental reactors |
Fig. -5 shows the maximum specific activity of protease in different bioreactors. Maximum activity was seen in cascade of STR’s of 4 and 6 series. In both the reactors highest production is achieved in 4th bioreactor and remained almost at steady state in 5th and 6th bioreactors. The maximum specific activity achieved is 13.7-14.0 units/ml from 4th reactor onwards and maintained at the same concentration of enzyme thereon in 6 series.
Table 1: Comparison of different bioreactors for the production of protease by Bacillus subtilis
| S.No. | Name of Reactor | Max. Specific activity (mmol/min/mg of protein) | % Increase |
| 1 | Shake flask | 4.79 | – |
| 2 | STR | 10.106 | 52% |
| 3 | Gradostat 4 | 13.71 | 38% |
| 4 | Gradostat 6 | 14.1 | 10% |
Table -1 shows the percentage of increase in protease production between different bioreactors. When compared to shake flask, STR had produced 52% more amount of enzyme. The percentage of protease production by Cascade of STR is approximately 38% more when compared with STR. The increase in enzyme production might be due to physiological state of the organism with respect to its origin15. The production achieved in 4th bioreactor on wards is at steady state and the organism metabolic activity remains same irrespective of number of bioreactors in the series.
Acknowledgments
Authors are grateful to Dr. J. S. Yadav, Director, IICT, for his encouragement.
References
- Aunstrup K., Microbiol., 5, 50 (1980).
- Michel C. F. and Stephen W. D., Enzymes, Protein hydrolysis: Encyclopedia of bioprocess technology, 2, 1074 (1999).
- Han S. J. and Chung S. C., Process Biochem., 40, 1263 (2005).
- Kembhavi A. A., Kulkarni A. and Pant A., Biochem. Biotechnol., 38, 83 (1993).
- Horikoshii K., Mol. Biol. Rev., 63, 735 (1999).
- Rao M. B., Tanksale A. M., Ghatge M. S. and Deshpande V. V., Mol. Biol. Rev., 62, 597 (1998).
- Leipner J. and Saller R., Drugs, 59, 769 (2000).
- Leipner J., Iten F. and Saller R., Biodrugs, 15, 779 (2001).
- Abdel-Naby M. A., Ismail A. M. S., Ahmed S. A. and Abdel Fattah A. F., Bioresource , 64, 205 (1998).
- Feng Y. Y., Yang W. B., Ong S. L., Hu J. Y. and Ng W. J., Microbiol. Biotechnol., 57, 153 (2001).
- Longo M. A., Novella I. S., Garcia L. A. and Diaz M., Biosci. Bioeng., 88, 35 (1999).
- Gupta R., Beg Q. K. and Lorenz P., Microbiol. Biotechnol., 59, 15 (2002).
- Lovitt R. W. and Wimpenny J. W., Gen. Microbiol., 127, 261 (1981).
- Govender S., Jacobs E. P., Leukes W. D. and Pillay V. L., Letters., 25, 127 (2003).
- Fredrickson A. G., J., 6, 835 (1992).
- Jetty A., Gangagni Rao A., Sarva Rao B., Madhavi G. and Ramakrishna S. V., Biochem. Eng. Q., 19, 179 (2005).
- Mabrouk S. S., Bioresource Technol., 69, 155 (1999).
- Clarence H. S., A practical Guide to Enzymology and Biochemistry, A Series of Monographs, 3, 27 (1985).
- Lowry O. H., Rosenbrough N. J., Farr A. L. and Randall R. J., Biol. Chem., 193, 265 (1951).
- Belmar-Beiny M. T. and Thomas C. R., Bioeng., 37, 456 (1990).
- Lovitt R. W. and Wimpenny J. W. T., Gen. Microbiol., 127, 269 (1981).

This work is licensed under a Creative Commons Attribution 4.0 International License.





