How to Cite | Publication History | PlumX Article Matrix
Lokeswari*, M. Narasimha Rao, K. Jaya Raju and C. Ayyanna
Center for Biotechnology, Department of Chemical Engineering, Andhra University, Visakhapatnam - 530 00, India
ABSTRACT: A comparative study on the production of Gallic acid using three different types of tannin rich substrates viz Anacardium occidentale, Caesalpinia digyna, Caesalpinia coriaria was conducted in the present work. Among these three substrates Caesalpinia digyna is richest source of Tannins as it contains 50% Gallotannins of total tannins, which is more than that of other two substrates (A. occidentale 26%, C. coriaria 43%). Some important parameters like substrate concentration, pH, temperature, inoculum level, fermentation time were optimized for the hydrolysis of Gallotannin from the above said three substrates by using Fusarium solani MTCC 350 which produces tannase enzyme when induced with 5gL-1 gallotannin. The highest yield of Gallic acid could be obtained from Caesalpinia digyna pod cover powder at substrate concentration 4 % (w/v), pH 5, temperature 35OC, inoculum level 8 ml, and fermentation time of 3 hours. Hence this method produces more yield of Gallic acid from Teri tannins and at the same time it is free from pollution.
KEYWORDS: Anacardium occidentale; Caesalpinia digyna; Caesalpinia coriaria; Fusarium solani; Gallic Acid; Tannin, Tannase
| Copy the following to cite this article: Lokeswari N, Rao M. N, Raju K. J, Ayyanna C. Comparative Study on Biotransformation of Tannin- Rich Agro / Forest Based Products into Gallic Acid by Fusarium solani MTCC 350. Biosci Biotech Res Asia 2006;3(2a). |
| Copy the following to cite this URL: Lokeswari N, Rao M. N, Raju K. J, Ayyanna C. Comparative Study on Biotransformation of Tannin- Rich Agro / Forest Based Products into Gallic Acid by Fusarium solani MTCC 350. Biosci Biotech Res Asia 2006;3(2a). Available from: https://bit.ly/3fHenE7 |
Introduction
Gallic acid, chemically known as 3, 4, 5 – Trihydroxy benzoic acid, a product of tannin hydrolysis, finds primary use as the basic intermediate for a very important anti – bacterial drug, Trimethoprim. It is also used in pharmaceutical industry, (Chadi et al., 1994), as astringent and as styptic agent (Mukharjee and Banerjee, 2003). Gallic acid esters also are used as antioxidant, in the manufacture of ordinary writing inks and dyes, as photographic developer, in the enzymatic synthesis of propyl gallate, in tannery industry for homogenization of tannins. The tannins are esters of gallic acid and are obtained from extraction of Teri pods, Tara pods, and Testa of cashew nuts. The tannins are also obtained from dried and powdered leaves of sumac shrub, tea leaves, oak bark, horse chest nut, myrobalan fruits, etc. (Nishizawa et al., 1983). Table -1 shows the % of gallotannin concentration in the plant sources. The Fig.-1 shows the pods of Tara,Teri and Testa of Cashew seeds.
Microorganisms have been reported to produce during fermentation the industrially important enzyme tannin acyl hydrolase (EC 3.1.1.20), which is capable of hydrolyzing tannins to Gallic acid (Lekha and Lonsane, 1997). Halsam and Stangroom (1966) resolved tannase into two separate enzymes – an esterase and depsidase with specificities to methyl gallate and m – digallic acid respectively. Tannase also finds use in wine making, beer chill proofing, and production of instant tea by solubilization of tea cream and in the pre – treatment of animal feed additives. The fungi, Fusarium solani MTCC 350 are used for the bioconversion of the tannin – rich substrates to gallic acid. Microbes are dependent on the affecting process parameters for their growth, and product yield and in turn depends on the enzyme synthesis by the microbe. In the present study the effects of the process parameters on tannase and gallic acid production by submerged fermentation were studied and their optimum levels were determined. The present work proposes to screen plants for economic source of gallotannins and to develop a process for the production of gallic acid using a spectrophotometric method. This would help the country to produce gallic acid form the natural resources not only to meet indigenous demand but also to export gallic acid to other developing nations.
In the present study tannin rich agro-residues comprising of powdered pod cover of Caesalpinia digyna, and Caesalpinia coriaria, powder testa of cashew seeds were used for carrying out submerged fermentation
Methods
Microorganisms and Growth
Fungal strain of Fusarium solani (MTCC 350) were procured from microbial type culture collection, Chandigarh, India. Stock cultures were grow an on 0.01% gallotannin supplemented potato dextrose agar slants and maintained at 4°C. The slants were sub – cultured routinely at an interval of 4 – 5 weeks.
Raw Materials
Caesalpinia digyna (Teri Pods) cover powder, Caesalpinia coriaria (Tara pods) cover powder and Anacardium occidentale (Testa) were dried and milled to get the particle size below 5 mm, 4 gm of tripod powder is taken in 100 ml water in the 250 ml conical flask and the flask was closed with cotton plug. Tannins were extracted by autoclave at 10 PSI for half an hour. After 30 min the extract was filtered through cloth. This extract was evaporated and the obtained powder form was used for the entire experiment.
Preparation of Spore Suspension
8 ml of sterile distilled water was taken in 50 ml conical flask. The mycelia of the slant cultures were scraped off in 2 ml of distilled water. The resulting spore suspension was mixed to obtain a uniform suspension. This suspension was then added to distilled water to give 10 ml of spore suspension for spore dilution. 50 ml medium is transferred to each of 250 ml conical flask and then sterilized. These flasks were inoculated aseptically with 2 ml of spore suspension prepared form the culture slants. These flasks were kept in a rotatory shaker (160 rpm) at 30 ± 2°C for 48 hours. After 48 hrs of incubation the fungal mycelia was washed thoroughly with distilled water for subsequent studies this inoculum was used by centrifuging at 5000 rpm for 15 minutes and wet mass is used as inoculum.
Estimation of Total Tannins
The estimation of tannin content was done following the protein precipitation method of Haggerman and Butler (1978). Bovine serum albumin (BSA) was taken as the standard protein. The total extractable tannin concentration of three substrates is Caesalpinia digyna (Teripods) 0.270 mg/ml, 52% (w/v), Caesalpinia coriario (Tara pods) 0.140 mg/ml ,43% (w/v), Anacardium occidentale (Testa) 0.075 mg/ml.26% (w/v).
Table 1: Tannin Plant Source
| Tannin type | Plant Source | Plant part used
for extraction |
Gallotannin content
(gm/100 gm dry wt.) |
| Caesalpinia digyna, | Teri tannins | Pods | 28 – 41% |
| Caesalpinnia coriaria | Tara tannins | Pods | 53 – 59% |
| Anacardium occidentale | Testa tannin | Testa | 25 % |
Preparation of Induced Inoculum
Tannase being an adaptive enzyme, preinduced inoculum is required to be prepared. The medium used for growing fungi, Fusarium solani was potato dextrose broth containing 0.5% gallotannin adjusted to pH 5.6.
Estimation of Gallic Acid in the Medium using Rhodanine
Kenneth, Hagerman, (1988) reported that the Gallic acid is assayed by using Rhodanine. The fermented broth is taken in centrifugation tube and centrifuged for 5 min. at 10,000 rpm. 0.025 ml of supernatant liquid is taken into 25 ml, stoppered volumetric flask. 1.5 ml of 0.0667% methanolic Rhodanine solution was added to that, after exactly 5 min. 1ml of 0.5 N aqueous KOH was added. After 2.5 min. the mixture was diluted with distilled water. 5-10 min. later the absorbance at 520 nm was read against reagent blank. The amount of Gallic acid can be obtained from Gallic acid standard curve.
Results and Discussion
In the present work studies on the gallic acid production from Teri pods, Tara pods and Cashew testa tannin using Fusarium solani (MTCC 350) were carried out. The effect of various parameters at different ranges were studied with different tannin containing substrates and their influence on the fermentation was discussed in this chapter. All the experiments were carried out in sequential order with optimized values. To find out the unknown concentration of Gallic acid produced after gallotannin hydrolysis with different tannin rich substrates and also at some important parameters a standard curve is prepared taking gallic acid of analytical grade. Effect of some important parameters on hydrolysis of gallic acid by using the above three substrates has been studied. Percent hydrolysis of gallotannin to gallic acid was calculated on the basis of tannin content of the substrates by the fermentation process.
 |
Figure 1: Tannin substrates |
Effect of Fermentation Time on Gallic Acid Production
In order to determine the optimum fermentation time for gallic acid production using F. solani MTCC 350, experiments were conducted. The results were tabulated in Table 2 and also Fig. 2. From the Fig 2 it can be observed that as the fermentation time increases, the gallic acid production was also increased. Maximum amount of gallic acid production was observed at 3 hours fermentation in the case of Teri pod tannins compared to other two substrates. Further increase in fermentation time does not increase the gallic acid production, instead there is a slight decrease. This decrease is probably due to the break down of gallic acid by the organism to carbon dioxide and water. Even though the gallic acid production was maximum at 3 hours, there was slight increase in the gallic acid production between 2 to 3 hours of fermentation. Hence, 3 hours was chosen as the optimum fermentation time.
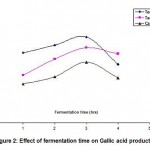 |
Figure 2: Effect of fermentation time on Gallic acid production |
Table 2: Comparison of the effect of some parameters on the hydrolysis of gallotannin to Gallic acid by fungi F. solani using three different substrates
| Parameters | Teri | Tara | Cashew |
| pods | pods | testa | |
| 1. Fermentation time (h) | |||
| 1h | 6.42 | 4.36 | 3.56 |
| 2h | 7.12 | 5.84 | 4.22 |
| 3h | 7.86 | 6.88 | 5.56 |
| 4h | 5.36 | 6.32 | 4.12 |
| 2. Substrate Conc %(w/v) | |||
| 2 % | 6.2 | 4.22 | 3.6 |
| 4 % | 8.48 | 5.20 | 4.66 |
| 6 % | 7.84 | 6.32 | 5.02 |
| 8 % | 7.62 | 5.24 | 5.86 |
| 10 % | 7.12 | 4.28 | 5.58 |
| 3. pH | |||
| 3 | 5.52 | 4.32 | 3.12 |
| 4 | 6.32 | 4.96 | 4.62 |
| 5 | 7.96 | 6.0 | 5.68 |
| 6 | 5.64 | 5.52 | 4.54 |
| 4. Temperature (°C) | |||
| 25°C | 6.88 | 4.94 | 3.02 |
| 35°C | 8.76 | 6.84 | 5.88 |
| 45°C | 7.76 | 5.28 | 4.36 |
| 55°C | 6.96 | 4.32 | 3.06 |
| 5. Inoculum level (v/v) | |||
| 2 ml | 5.29 | 3.12 | 3.02 |
| 4 ml | 6.72 | 4.12 | 6.22 |
| 6 ml | 7.84 | 7.82 | 4.42 |
| 8 ml | 8.44 | 6.44 | 4.26 |
| 10 ml | 7.76 | 5.36 | 4.26 |
Effect of Substrate Concentration on Gallic Acid Production
The influence of substrate concentration on the production of gallic acid was studied by varying the substrate concentrations viz 2, 4, 6, 8, 10 % (w/v) and the results were tabulated in Table -2 and also shown in Fig. -3. It can be observed from the results, the optimum substrate concentration was 4% for Teri pods, 6% for Tara pods and 8% for Cashew Testa and the maximum conversion was obtained. The variation from the reported results may be due to the variation of Gallotannin concentration in different substrates. Teri pods are rich source of Gallotannins than Tara pods and Cashew Testa. (Ikeda et al., 1983) reported the optimal substrate concentration as 5% (w/v) working on Casesalpinie spinosa.
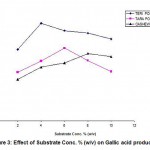 |
Figure 3: Effect of Substrate Conc. % (w/v) on Gallic acid production |
Effect of pH on Gallic Acid Production
To find out the effect of pH on the gallic acid production, experiments were conducted by varying the pH of the medium from 3 to 6 with an increment of 1 using 0.1N hydrochloric acid and 0.1N sodium hydroxide. The results were tabulated in Tabel 2 and also shown in Fig 4. The effect of PH on the production of gallic acid was found to be very important since the PH profile gives a sharp peak. From the Fig. -4 it was understood that the production was low at PH 3 and it increased exponentially up to PH 5 and there after it started to decline. Hence, PH 5 was taken as the optimum for gallic acid production. At pH 5 the Teri pods yields maximum gallic acid compared to other two substrates, Tara pods and Cashew testa. Optimum pH was reported to be 6.0 by ( Pourrat et al, 1982 ) working on a different organism i.e. A. niger.
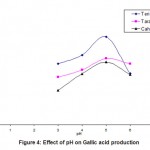 |
Figure 4: Effect of pH on Gallic acid production |
Effect of Temperature on Gallic Acid Production
Experiments were conducted on gallic acid production by varying the temperature from 250C to 550C with 10 increment using three substrates and the results were shown in Table -2 and also shown in Fig. -5. The temperature had a profound effect on gallic acid production. From the Fig., it can be seen that the optimum temperature for gallic acid production was at 35OC. Further increase in the temperature resulted in decreasing gallic acid yields, which could be due to the decreased activity and viability of fungi F. solani. This decreased activity and viability may be due to the increase in temperature in the microenvironment. Optimal Temperature was reported to be 55OC by (Abdel and Sherif, 1999) working with immobilized enzyme by using Aspergillus oryzae.
 |
Figure 5: Effect of temperature on Gallic acid production |
Effect of Inoculum Level on Gallic Acid Production
The effect of inoculum level on Gallic acid production was studied by using different inoculum levels ranging from 2 ml to 10 ml in 50 ml of medium with 2 ml increment using three different substrates. The results obtained were given in Table 2 and also shown in Fig. -6.
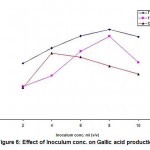 |
Figure 6: Effect of Inoculum conc. on Gallic acid production |
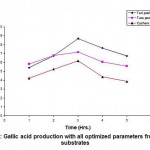 |
Figure 7: Gallic acid production with all optimized parameters from three substrates |
Gallic Acid Production with all Optimal Conditions using Three Substrates
The production of Gallic acid from, gallotannins using Fusarium solani were carried out using three types of substrates – Caesalpinia digyna (Teri pod), Caesalpinia coriaria (Tara pod), Anacardium occidentale (Testa). Using the optimal conditions in the submerged fermentations of these three substrates resulted in the higher yields of Gallic acid. This experiment was carried out using all the already estimated optimized values.
The optimal conditions for Teri pod tannins are substrate concentration 4% (w/v), pH 5, temperature 35OC , inoculum level 8 ml, fermentation time 3 hours. With the above optimal conditions, Teri pod exhibited maximum bioconversion of gallic acid, 8.68 mg/ml a time period of after 160 min.
The optimal conditions for Tara pod tannins are substrate concentration 6 % (w/v), PH 5, temperature 35OC , inoculum level 6 ml, fermentation time 3 hours. With the above optimal conditions, Tara pod exhibited maximum bioconversion of gallic acid, 7.16 mg/ml a time period of after 160 mins.
The optimal conditions for Cashew Testa tannins are substrate concentration 8 % (w/v), pH 5, temperature 35O C, inoculum level 4 ml, fermentation time 3 hours. With the above optimal conditions, Cashew Testa exhibited maximum bioconversion of gallic acid, 6.18 mg/ml a time period of after 160 min. The results obtained were presented in the Table 3. The results obtained indicate that, all the three experimental substrates exhibited maximum hydrolysis at initial pH 5, temperature 35OC,Fermentation time 3 hours, The pH of the reaction medium showed an increase towards acidic side as the hydrolysis progressed due to accumulation of gallic acid. Similar variation in gallotannin source was also observed at different substrate and inoculum concentrations. Teri pods exhibited peak bioconversion at 160 min.
Table 3: Gallic acid production at various optimal parameter conditions using three different types of substrates
| Optimal | Teri | Tara | Cashew |
| parameters | pods | pods | testa |
| Fermentation time | |||
| 1 to 4 hrs. | 3 hours | 3 hours | 3 hours |
| Substrate conc. | |||
| 2% to 10%. | 4 gm | 6 gm | 8 gm |
| pH 3 to 5 | 5 | 5 | 5 |
| Temperature | |||
| 25oC to 550 C. | 35oC | 35oC | 35oC |
| Inaculum level | |||
| 2 ml to 10 ml (v/v). | 8 ml | 6 ml | 4 ml |
| Yield of Gallic acid | 8.68 | 7.16 | 6.18 |
| mg/ml | mg/ml | mg/ml. |
Table 4: Gallic acid production using all optimized parameters from Teri pods, Tara pods, and cashew Testa tannins
| Concentration of Gallic acid mg/ml | |||
| Time in hours | Teri pods | Tara pods | Cashew testa |
| 1 hour | 5.41 | 5.82 | 4.22 |
| 2 hour | 6.72 | 6.76 | 5.24 |
| 3 hour | 8.68 | 7.16 | 6.18 |
| 4 hour | 7.62 | 6.06 | 4.38 |
| 5 hour | 6.72 | 5.58 | 3.86 |
Gallic Acid Production at Optimal Conditions
The experiments were conducted at all the optimal conditions using Fusarium solani and three substrates. The results shown in Table 4 and in Fig. 7 indicate that all the three experimental tannin rich substrates exhibited maximum production of gallic acid after 3 hours of the fermentation time. Teri tannins showed the highest yield of Gallic acid 8.68 mg/ml when compared to Tara and Cashew Testa tannins.
Conclusions
The present work has been taken up with a view to explore the importance of tannin rich substrates viz Teri pods, Tara pods, and Cashew Testa for the production of Gallic acid by using fungal mycelia Fusarium solani. The concentration of Gallic acid produced at all optimized parameters from Caesalpinia digyna pod cover containing tannins is 8.68 mg/ml which corresponds to 81.1% yield, Caesalpinia coriaria pod cover powder containing tannins is 7.16 mg/ml which corresponds to 72% yield, and Anacardium occidentale testa powder containing tannins is 6.18 mg/ml which corresponds to 58% yield. Thus this investigation proposes that the Caesalpinia digyna (Teri pod), a tannin-rich forest product can be used as substrate for maximum production of Gallic acid.
References
- Abdel., M.A., Sherif. A.A.: Gallic acid production from newley isolated organisam. J. Appl. Microbio., (1999).
- Haggerman,A .E.,Butler,L.G., Protein precipitation method for determinations of Tannins. J. Agric. Food. Chem. 26, 809-812 (1978).
- Hadi, T.A., Banerjee, R., Bhattacharya, B.C.,Optimization of tannase, Biosynthesis by a newly isolated Rizopues oryzae. Bio-process Eng ., 11, 239 – 243 (1994).
- Knenneth H., Hagerman, Determination of galotannin with Rhodanine (1988).
- Lekha, P.K., Lonsane, B.K., Production and application of tannin acyl hydrolase; state of the art. Ad. Appl Microbial. 44, 215-260 (1997).
- Nishizawa, M., Yamagiashi, T., Nonka, G, and Nishiokal I. Tannins and related compounds:part 9: isolation and characterization of polygalloyglucoses from Turkish galls (quercer infectoria), J. Chem. Soc. Perkin-Tranes 15 : 961 (1983).
- POurrat, H., Regrat. F., Pourrat., A., and Jean, D. Production of Gallic acid from Tara by a strain of Aspergillus niger. J. Ferment Technol., 63: 401 (1982).

This work is licensed under a Creative Commons Attribution 4.0 International License.





