How to Cite | Publication History | PlumX Article Matrix
Effect of Bovine Blood Fractions on the Infectivity of Theileria parva to Bovine Blood Lymphocytes
Esther Kibuka-Sebitosi*1 and Godwin P. Kaaya2
1Centre for African Renaissance Studies (CARS), University of South Africa (UNISA), 287 Skinner Street, Pretoria, P.O. Box 392 UNISA 0003, Pretoria, South Africa.
2Department of Biology, University of Namibia, Private Bag 13310, Windhoek, Namibia.
ABSTRACT: An assay for feeding ticks on whole blood and blood fractions and assessment of its ability to transmit Theileria parva parasites in vitro was established. Bovine blood was separated into plasma and erythrocytes by centrifugation and then fed to ticks in vitro. Results indicated that plasma alone was unable to support the development of T. parva sporozoites and their infectivity to peripheral bovine blood lymphocytes, resulting in only 0.07% infection rates, compared to 1.02% for erythrocytes and 20% for whole blood. The control ticks that fed in vivo on rabbits produced the highest infection rates (67.9%). Based on observations of the number of schizonts, as evidence of proliferation of peripheral blood bovine lymphocytes, it is deduced that In vitro infections of lymphocytes were successfully carried out with sporozoites obtained from ticks fed in vitro. This technique may be useful in future transmission experiments for vector-borne diseases.
KEYWORDS: Artificial/Membrane Feeding; East Coast Fever; In vitro Transmission; Rhipicephalus appendiculatus; Theileria parva; Ticks; Theileriosis
| Copy the following to cite this article: Kibuka-Sebitosi E, Kaaya G. P. Effect of Bovine Blood Fractions on the Infectivity of Theileria parva to Bovine Blood Lymphocytes. Biosci Biotech Res Asia 2006;3(2a). |
| Copy the following to cite this URL: Kibuka-Sebitosi E, Kaaya G. P. Effect of Bovine Blood Fractions on the Infectivity of Theileria parva to Bovine Blood Lymphocytes. Biosci Biotech Res Asia 2006;3(2a). Available from: https://bit.ly/39VMM0F |
Introduction
Ticks are obligate hematophagous ectoparasites of terrestrial vertebrate hosts, including cattle, goats and wild animals. They utilize blood for growth, general metabolism and reproduction. Vitellogenesis and oviposition in females, for instance, cannot occur unless females have taken a blood meal (Akov, 1982). Ticks take several milliliters of blood depending on whether they are “hard” or “soft” tick, and on their developmental stage. Blood digestion is slow and intracellular (Arthur, 1965; Balashov, 1972; Araman, 1979; Akov, 1982). Adult female ixodids may ingest ten times their body weight of blood (Alan Young, Pers. com.).
Theileriosis is the name given to infections caused by several species of Theileria (Theiler, 1911). Theileria parasites are of great economic importance in many parts of the world where they cause disease to domestic animals (Purnell, 1977) -the two most important in Africa being Theileria parva and Theileria annulata. These diseases have been given several names in different countries (Norval et al., 1992). Theileria parva infections are referred to as East Coast Fever, Corridor disease or January disease, while T. annulata infections are called tropical theileriosis. Theileria parva is transmitted mainly by the brown ear tick Rhipicephalus appendiculatus Neumann, 1901 (Norval et al., 1992). During feeding, ticks inject T. parva sporozoites into the blood of cattle, resulting in progressive lymphoproliferative disease initiated by the transformation of parasitized mononuclear cells in the lymph nodes. Once inside the lymphocytes, the sporozoites develop into multinucleate macroschizonts.
Despite several in vitro tick studies (Stiller & Coan, 1995; Kuhnert, 1996; Kuhnert et al., 1998; Neese et al., 2000; Rechav et al., 2000) and transmission experiments (Voigt, 1993; Humphrey-Smith, 1993; Waladde et al., 1995; Inokuma & Kemp, 1998; Kimbita & Silayo, 1997; Musyoki et al. 2004), little is known about the role of the different blood fractions ingested by the tick on the development of the parasite Theileria parva. In this study, the role of the blood fractions in the development of the parasite T. parva, and the subsequent invasion of bovine host lymphocytes was investigated. Through this study a technique that can be used to investigate infectivity of sporozoites in transmission experiments in vitro was also developed.
Materials and Methods
Ticks and T. parva parasites: Theileria parva (Muguga stock) and the tick R. appendiculatus maintained in the laboratory at the International Livestock Research Institute (ILRI) in Nairobi, Kenya, were used in these studies.
Tick attachment, preparation of ear wash, and ear-wash composition
Two ear wash extracts (methanol and diethyl ether) were compared in their composition and enhancement of tick attachment. Rhipicephalus appendiculatus adults which normally attach to the ears to feed, hence the name brown ear tick, were left to feed for 4 days. The ears were washed using two solvents- methanol and diethyl separately, in order to extract tick attractants. A large volume of the diethyl ether (1 litre) was used to wash the two cattle ears and the mixture was concentrated by evaporating off the ether in a rotor evaporator. Several precautions were taken, since ether is volatile and inflammable. The evaporator (Rotarvapor RE) was particularly selected for this purpose and a warm water bath was used to heat the ether. The apparatus had a Liebig condenser for condensation of the ether. The excess vapor was cooled down in an “ice cold trap” dry ice. The system was put in a fume hood that created a vacuum, by blowing air from the room out through a vent. The concentrate was divided into aliquot parts and kept at -20oC until needed. The methanol concentration was carried out in a 55oC water bath.
An attempt was made to analyze the possible components of the ear wash extract using thin liquid chromatography (TLC) with a solvent system of the ratio 1 ethyl acetate: 4 hexane. Under ultraviolet light, three bands were observed, one at methanol and at each diethyl ether (color pink). The diethyl ether had several compounds extracted compared to methanol. These were later found to be phenols, salicylaldehyde, 4-cresol, 2,6-dichlorophenol, 2,6-dibromophenol and 2-bromo-6-chlorophenol, responsible for the attraction of ticks. The extract was utilized as attractants in the in vitro feeding chambers. The extracts from the two solvents were compared for tick attachment. Diethyl ether proved to be the most effective solvent in that more than 90% nymphal attachment was observed compared to methanol of 40-50%.
Semiochemicals
The membrane was made attractive for tick attachment by providing a combination of sui chemicals (semiochemicals of hosts and tick fecal material), a temperature of 37oC and relative humidity (Rh) of 80-85% were provided as physical stimuli. The external surface of the membrane was therefore treated with cattle/tick ear wash and then sprinkled with tick fecal material collected from the tick incubation tubes similar to Walladde et al. (1996).
The in vitro Tick Feeding System
The in vitro tick feeding system, similar to that described by Walladde et al., (1993, 1995, 1996), with minor modifications (Fig. -1), was used to study the role of the blood meal fractions on infectivity of blood lymphocytes by T. parva and on the maturation of the sporozoites.
Detoxification of the Membranes
The toxic substances in the chambers (mainly the glue dissolved in chloroform) were removed by rinsing each of them in a water bath at least 3 times at 80oC for at least 8 hr at a time, after which the chambers were then dried under a hood for 12 hr. The interiors of the chambers were sterilized with a 1:1:1 mixture of gentamycin, penicillin/streptomycin and fungizone. The external aperture was covered with zinc oxide plaster, which kept it sealed except when blood was introduced or changed.
Tick Application
Following the chamber preparation, the ear wash concentrate was carefully daubed onto the membrane and a cluster of 500-1000 nymphs was applied onto the treated membrane surface. Adult ticks were first sexed in an electronic tick trough heated at the sides to prevent them from escaping before they were applied to the membrane at a 1:1 sex ratio. To avoid overcrowding and piercing the membrane, which could lead to chamber leakage, only 60-100 adults were used.
Maintenance of Chambers
During the experimental period, blood was changed twice daily at 08:00 and 17:00 hrs, under sterile conditions obtained by wiping the hood with 70% ethanol. The chambers were rinsed with normal saline (9.0 g/l NaCl) before fresh blood was put into them. Any chambers with very dark red or black blood suspected of contamination were rinsed with a mixture of penicillin/streptomycin, gentamycine and fungizone. The blood was introduced into the chambers by opening the zinc oxide plaster on top of the blood chamber (Fig. – 1) under a sterile hood, previously swabbed with 70% ethanol. The aseptically – collected heparinized cattle blood (10-12ml) was pipeted into the chamber using a pipette aid (Drummon, UK) fitted with a 10 ml disposable plastic pipette (Costar, Cambridge, Massachusetts, USA) and the aperture was then immediately sealed with the plaster. The pipette tip was placed at the side of the chamber when dispensing blood to avoid piercing through the membrane. The whole feeding system was placed in an incubator at 37oC with a 5% CO2 flow. The incubator had a Rh of 80-85%, obtained by placing an open tray containing saturated potassium chloride solution inside (Alan Young, Per. com).
Separation of Blood Components for in vitro System
Blood samples from cattle (6 – month – old Borans) were collected in heparin (1:5000 units) by jugular venipuncture and divided into samples of whole blood, plasma and red blood cells. The plasma was obtained by centrifugation of whole blood (1000 x g; 4oC) in a heraeus sepatech megafuse. After removing the clear plasma, the cells were cleared of the buffy coat layers by sucking with Pasteur pipettes. Some of the blood was left uncentrifuged. The cells, whole blood, as well as the plasma, were all warmed to 37oC in a water bath for 15 min before they were transferred into the feeding chambers. Three replicas comprising 2 chambers each were set up for 3 different tick batches. The chambers were maintained at 37oC, 5% carbondioxide and 80-85% Rh. The relative humidity was obtained by keeping a tray of saturated potassium chloride in the incubator throughout the experiment. The blood samples were changed twice daily and the chambers were rinsed with normal saline between the changes.
In vivo Feeding of Ticks
For comparison with the in vitro system, ticks were also fed in vivo on rabbits. In short, about 100 males and females in ear bags were fed on rabbit ears for 4 days. The rabbits were kept in cages and a leather collar was put around their necks to prevent them from detaching the ear bags. To ensure homogeneity in the samples, the ticks put on the rabbits were from the same batches as those applied in vitro.
Selection of Tick Batches
Ticks were infected as nymphs by letting them feed on artificially-infected cattle when the erythrocyte piroplasm counts were high (at least >10%). Engorged nymphs were maintained at 23-250 C and 80 % Rh and allowed to molt to adult stage. The adult ticks used in determining the sporozoite infectivity of the peripheral blood lymphocytes (PBLs), were first screened for their infection rates (percentage of infected ticks per number examined) by Fuelgen staining of whole salivary glands after the ticks had fed on rabbits for 3 days. This was necessary in order to standardize the number of infected acini used in the interaction of the sporozoites with the PBLs. Tick batches that showed at least 80% infection rates were used. The abundance (the mean number of infected acini per tick) was calculated from the infection rates. Since the required number of infected acini was 1000 and the abundance was 94.6, the number of ticks that were to be dissected was obtained by dividing the required number of infected acini by the abundance (1000/94.6). Thus, only 11 ticks were required for dissection.
Synchronization of in vitro and in vivo feeding systems
In order to compare sporozoite maturation and their ability to infect lymphocytes, the two feeding systems, the in vitro and the in vivo, were synchronized so that fresh sporozoites were obtained on the same days. This was carried out by applying at least 60-100 adults (both sexes) in the feeding chambers at least 24-48 hr prior to the application of ticks on the rabbit ears (Table 1). This time period allowed the in vitro-fed ticks to attach, since the ticks put on the rabbits attached faster. In the feeding chambers, plasma, whole blood or erythrocytes were separately added and the ticks were allowed to feed for 4 days.
Tick Dissections for Sporozoites
Ticks were removed with a pair of forceps, washed in detergent for a few minutes, followed by a rinse in distilled water. They were then sterilized by rinsing in 70% ethanol and finally in RPMI 1640 cell culture medium and N-(2-Hydroxyethyl) piperazine N’-2-ethane-sulfonic acid (HEPES) medium. The salivary glands were dissected out under sterile conditions in a Laminar Flow Hood as described in Sebitosi et al., (1998).
Handling of T. parva Sporozoites for in vitro Infection
Viable sporozoites (Muguga stock) were prepared from the salivary glands of R. appendiculatus adults fed for 4 days on different blood fractions or on rabbits, as described above. They were dissected in a special medium consisting of RPMI 1640 + HEPES , L-glutamine, fetal calf serum (FCS)(5%), gentamycin and penstrep (antibiotics) 5 ml to keep the parasites alive. The sporozoite containing salivary glands from the three groups were separately homogenized in the above medium in a 0.1ml homogenizer and the volume was made up to 0.1ml. All these processes were carried out under a hood. The homogenate was centrifuged at 150 x g for 5 min to remove salivary gland debris, and the supernatant was used without further purification in the infection of lymphocytes.
Preparation of PBLs for in vitro Infection
Cattle blood was obtained from uninfected 6-9 months old Boran cattle (Bos indicus). It was drawn from the jugular vein with a 16-gauge needle into a syringe containing an equal volume of Alsevers solution and heparin. The cattle were kept on a farm free from East Coast fever, and brought to the ILRI farm at the age of 1-2 weeks, where they were maintained. They were screened for antibodies to T. parva using the indirect immunofluorescent antibody test (IFAT), immunoblotting and indirect enzyme linked immuno sorbent assay (ELISA) (Goddeeris et. al., 1982; Katende et. al., 1990) before the experiment started.
Processing of PBLs
The preparation of PBLs was carried out according to the technique of Goddeeris et al., (1982) and Katende et al. (1995), with minor modifications, in a horizontal laminar – airflow hood. Using a pipette, an aliquot of 30ml of blood was carefully layered on 20ml Ficoll Hypaque gradients contained in a 50ml polypropylene conical tube and centrifuged at 900 x g for 30 min in a Beckman centrifuge without brakes at room temperature (24oC). The centrifugation, without brakes, ensured that the cells settled properly.
Table 1: Synchronization of the in vivo and in vitro feeding systems for Rhipicephalus appendiculatus
| Day |
In vitro |
Activity |
In vivo |
| 0 | Ticks applied on feeding chambers plasma, cells and whole blood from cattle added |
–
|
|
| 1 | Ticks attachment and feeding | Ticks applied on rabbit ears
|
|
| 2-3 | Feeding of ticks on membrane
|
Feeding of ticks on rabbit ears | |
| 4 | Removal of ticks from membrane
|
Removal of ticks from rabbit ears
|
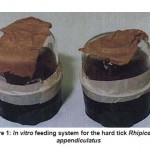 |
Figure 1: In vitro feeding system for the hard tick Rhipicephalus appendiculatus |
 |
Figure 2: Light microscope photograph of uninfected bovine lymphocytes obtained from in vitro infection of lymphocytes with sporozoites derived from R. |
 |
Figure 3: Theileria parva schizonts obtained from in vitro infection of lymphocytes with sporozoites derived from R. |
The PBLs were aspirated from the interface using a pipette and mixed with an equal volume of Alsevers solution in a 50ml polypropylene conical tube. The cells were pelleted by centrifugation at 450 x g at room temperature for 10 min. The PBLs were then washed three times in 50 ml Alsevers solution for resuspension and centrifugation at 180 x g for 10 min, to remove the platelets. The final wash was carried out in an L-15 culture medium. Finally, the pellet was resuspended in the L-15 medium, the composition of which was as follows: L-15 medium, FCS (20%), tryptophosphate broth (10%), 20mm HEPES, L-glutamine (200mM), penistrep and gentamycin (50mg/ml).
Standardization of Lymphocytes
Twenty microliters (20µl) of the cell suspension was added to 180µl trypan blue mixed on a vortex mixer (Type 167000), and the cells counted in a hemocytometer.
In vitro infection of lymphocytes with T. parva sporozoites
The infection of lymphocytes with T. parva sporozoites was carried out in a 96-well cell culture plate (Costar). A 100 µl sporozoite suspension at a concentration of 100 acini/well, for maximum infection, was mixed with a similar amount of PBLs and incubated at 37oC in a 5% CO2/air mixture for 1 hr to interact freely. The plates were then removed from the incubator and 200µl of the mixture was transferred into a 24-well cell culture plate (Costar) containing 500 µl feeder cells in RPMI + 10 % FCS. The plates were set up in duplicates and incubated at 37oC in a 5% CO2/air mixture.
The sporozoites and the lymphocytes were left to interact for 10 days with constant microscopic examination to check for schizonts. From the start of the neutralization assay (day 0) up to day 4, the mixture was left alone without disturbance. On day 5, 1 ml of the medium was removed and replaced with fresh medium (L-15 plus 20% FCS) containing 1 % mycostatin and 0.1 % gentamycin. The cells were left undisturbed for day 6 and day 7, and on day 8 cytospins were made.
Checking for Sporozoite Infectivity in Lymphocyte
Cytospins were necessary to produce flat cells on a glass slide for easy counting. Using a 1 ml pipette, the contents of each well were mixed and aliquots from 2 wells were transferred into a glass tube (Röhren Tubes, Sarstedi, Germany) and centrifuged at 150 x g for 5 min at 0oC to settle the cells without pelleting them. Fifty microliters of the supernatant was resuspended in a similar amount of PBS and vortexed (Super-mixer). In the cytospin, a volume of 50µl of the cell suspension was centrifuged at 70 x 10 rpm using low acceleration for 6 min. This was done on glass slides, which were lined with filter- paper cards. The slides were removed from the cytospin, air – dried for 2 min, fixed in methanol (5 min) on a rack and then Giemsa-stained (30 min). They were then washed in distilled water and examined under the microscope for the presence of intralymphocytic schizonts.
Schizont scores – All the slides were viewed carefully for schizonts. Both the number of cells in the field and the infected ones were counted and the percentage of infected cells (schizonts) was calculated out of a total of 400 cells.
Results
The use of aggregation pheromones and semiochemicals made from ear wash extraction and tick fecal material enhanced tick attachment to the membranes. Freshly prepared ear wash gave the best attachment results. Compared to methanol, diethyl ether proved to be the most effective solvent in that more than 90% nymphal attachment was observed compared to methanol of 40-50% tick attachement.
About 70-80% of ticks feeding on whole blood attached within 24-48 hr. In the erythrocyte or cell – feeding chambers, there was about 90% attachment of ticks within 24 hr. The ticks fed well and took on a characteristic reddish color, which distinguished them from all the other adults. The cells produced the best attachment, seen as “cones” on the membrane in the first 24-48 hrs. The in vivo (rabbit) feeding groups also had very good attachment (about 90-100 %) and fed well. The plasma however produced fewer attachments within the first 24-48 hrs.
The infection rates of T. parva sporozoites in the ticks used to infect the cattle were at least 80%. The abundance was 94.6 and intensity 118.25. The total acini examined were 2,838 and total acini used were 1000 extracted from a mean of 11 ticks per replicate. The total ticks per treatment were equal to the total acini required divided by abundance (a value of 10.57) or 11 ticks.
The lymphocytes (PBLs) that were not infected by the T. parva sporozoites were oval in shape (Figure 2). Those that were parasitized had an expanded nucleus and cytoplasm, and the schizonts were visible as pink dots in a blue cytoplasm (Figure 3). The sporozoites from the batch were viable, as predicted. The sporozoite infectivity was highest in the ticks fed on rabbits (67.9%), followed by whole cattle blood given in vitro 20%, erythrocytes (1%), and almost no infectivity was perceived in the plasma-fed ticks (Table 2).
Challenges of in vitro Feeding
The in vitro feeding chambers containing plasma had frequent leakages and self-sealing occurred. They also showed slow and poor tick attachment and over 20% adult mortality. The Baudruche membrane appeared bleached. The feeding chambers were also problematic in that there was frequent fungal infection and leakages. The in vitro system can be optimized to use blood from slaughterhouses thereby reducing the use of laboratory animals.
Discussion
The entry of sporozoites into the bovine lymphocytes during tick feeding is an important stage in the transmission of theileriosis. Once the tick feeds, it takes in a lot of piroplasms (about 1 million; Allan Young personal communication) whose development depends upon several midgut factors that the parasite encounters during its development-such as lectins and enzymes among others. Since the midgut factors are blood-meal-induced, the resultant infections would depend upon the type of host blood meal taken at the time of infection. Using the in vitro system and tissue culture techniques to study the various fractions of the blood meal and subsequent infectivity, some insight into the blood meal components influencing parasite development was gained.
The use of odour baits, aggregation attachment pheromones and /or semiochemicals in insects and ticks seems to be a natural phenomenon. The combination of host hair, tick fecal material, carbon dioxide and ear washings extract used in this study, enhanced the nymph attachment and is in agreement with other reports (Voigt et al., 1993; Waladde et al., 1995; 1996; Kuhnet, 1996).
Table – 2: Influence of blood meal fractions on the sporozoite infectivity of T. parva in peripheral blood bovine lymphocytes (PBLs)
| Tick feeding
(Blood meal) |
Sporozoite
Infectivity (%) |
Remarks |
| In- vivo (rabbit) | 67.99% | Almost all cells were infected with the parasite. Schizonts clearly stained blue with pink dots. |
| Whole blood (cattle) | 20.0% | Reasonable number of schizonts were visible |
| Cells | 1.02% | Extremely few schizonts observed. |
| Plasma | 0.070% | Some cells showed proliferation but few schizonts were observed. |
In some species of Amblyomma, the males produce the aggregation attachment pheromone (Rechav et al., 2000). Odour and tactile stimuli therefore seem to play an important role in tick attachment to artificial feeding chambers, as in the natural habitants. Whole blood from cattle supplied in vitro resulted in the highest level of sporozoite infectivity, followed by the erythrocytes, whereas the plasma resulted in almost no parasite infectivity.
Furthermore, ticks that were fed plasma did not attach effectively and detached frequently. This may have been caused by the lack of a vital factor for attachment in the plasma. There was, however, a good attachment in those ticks fed on a whole-blood meal. The erythrocyte-fed ticks, on the other hand, displayed a high number of attachments, implying that it is perhaps the erythrocytes that attract the ticks to attachments. However, the erythrocytes alone, despite attracting large numbers of ticks to attach, were unable to support a high number of mature, viable sporozoites ( 1% compared to 20% in the case of a whole blood meal) to infect the lymphocytes and eventually transform into schizonts.
The presence of erythrocytes therefore seems to be necessary in T. parva maturation. The mechanism that limited the sporozoite entry into the lymphocytes, especially in the case of plasma – fed ticks (as evidenced by the lack of schizont parasitosis) therefore needs further study. It is likely that vital ingredients in the plasma that are important in the sporozoite maturation process and establishment were lacking. This could have prevented the sporozoite in the vector from maturing to a crucial stage necessary for penetrating the lymphocytes, or the receptor-ligand interaction between the parasite and the lymphocytes might have been inhibited.
In this study, the in vivo system has been demonstrated to far supersede the in vitro system in sporozoite infectivity, as is evident in the high parasitosis among the rabbit-fed ticks compared to the whole-blood-fed ticks in vitro. This is the first time that sporozoites have been obtained from ticks in vitro and used to infect lymphocytes in vitro. Unlike the in vitro systems developed by others (Stiller and Coan 1995; Kuhnert, 1996; Kuhnert et al., 1998; Musyoki et al., 2004), this particular system was made from baudruche membranes with pheromones from tick feaces and ear wash. It is an important new avenue opened for the study of pathogen transmission, and is significant from the standpoint of evaluating potential immunogens in controlling the disease by vaccination against the parasite. It is concluded that the cells together with the plasma constitute the favorable environment in which sporozoites mature and are able to infect. If sporozoites do not mature to a certain critical stage, then they lack the necessary receptors to bind and penetrate into the lymphocytes.
Acknowledgements
We wish to acknowledge the assistance of the members of the tick Unit at the International Livestock Research Institute (ILRI), Nairobi, Kenya and those of Lab 5 in the preparation of the Theileria parva infections in cattle and ticks. Special thanks go to Dr. Antony Musoke, Prof. R.I.S.Agbede, Mr. Steven Mwaura and Mr. John Tangus. For the cell culture techniques, we acknowledge the assistance of Dr. Paul Spooner, Dr. Subash Morzaria, Mr. James Gachanja and Juma.
References
- Akov, S. Blood digestion in ticks. Physiology of ticks (ed. by F.D. Obenchain and R. Galun), 197-211. Pergamon Press, Oxford (1982).
- Araman, S.E. Protein digestion and synthesis in ixodid females. Recent advances in acarology (ed.by J.G. Rodriguez). 1. 385-395. Academic Press, New York (1979).
- Arthur, D.R. Feeding in ectoparasitic Acari with special reference to ticks.Advances in Parasitology 3, 249-298 (1965).
- Balashov, Y.S. Blood sucking ticks (Ixodidae).Vectors of disease of man and animals. Miscellaneous Publications of Entomological Society of America, 8, 161-362 (1972).
- Goddeeris, B.M., Katende, J., Irvin, M A.D. & Chumo, R.S.C. Indirect fluorescent antibody test for experimental and epizootiological studies on East coast fever (Theileria parva) infection in cattle. Evaluation of a cell culture schizont antigen fixed and stored in suspension. Research in Veterinary Science, 33, 360-365 (1982).
- Humphrey-Smith, I., G. Donker., A. Turzo., C.Chastel & Schmidt- Mayerova, H. Evaluation of mechanical transmission of HIV by the African soft tick, Ornithodoros moubata. AIDS, 7, 341-347 (1993).
- Inokuma, H. & Kemp, D.H. Establishment of Boophilus microplus infected with Babesia bigemina by using in vitro feeding technique. Journal of Veterinary Science, 60, 509-512 (1998).
- Katende, J.M., Goddeeris, B.M., Morzaria, S.P., Nkonge, C.G. & Musoke, A.J. Identification of a Theileria mutans-specific antigen for use in an antibody and antigendetection ELISA. Parasite Immunology, 12, 419-433 (1990).
- Kimbita, E.N & Silayo, R.S. Use of an in vitro infectivity assay in comparison with histological techniques in the study of Theileria parva sporozoites maturation. Veterinary Parasitology, 70, 83-97 (1997).
- Kuhnert, F. Feeding of hard ticks in vitro: New perspectives for rearing and for the identification of systemis Acaricides. Altex, 13, 76-87 (1996).
- Kuhnert, F., Issmer, A.E. & Grunewald, J. Partly automated in vitro feeding of adult Amblyomma hebraeum. Alte, 15, 67-72 (1998).
- Musyoki, J.M., Kiara, H.K. & Kokwaro, E.D. Comparative studies on the infectivity of Theileria parva in ticks fed in vitro and those fed on cattle. Experimental and Applied Acarology, 32, 51-67 (2004).
- Neese, P.A., Soneshine, D., Kallapur, V.L., Apperson, C.S & Roe, R.M. Absence of insect juvenile hormone in the American dog tick, Dermacentor variabilis (Say) (Acari:Ixodidae), and in Ornithodoros parkeri Cooley (Acari:Argasidae). Journal of Insect Physiology, 46, 477-490 (2000).
- Norval, R.A.I., Perry, B.D. & Young, A.S. The epidemiology of Theileriosis in Africa. 481, Academic Press (1992).
- Purnell, R.E. East coast fever: some recent research in East Africa. Advances in Parasitology, 15, 83-132 (1977).
- Rechav, Y., Drey, C., Fielden, L.J. & Goldberg, M. Production of pheromones by artificially fed males of the tick Amblyomma maculate (Acari:Ixodidae). Journal of Medical Entomology, 37, 761-765 (2000).
- Sebitosi, E.N.K., Kaaya, G.P., Young, A.S. & Agbede, R.I.S Lectins in the brownear tick Rhipicephalus appendiculatus. International Journal of Acarology, 24, 159-163 (1998).
- Stiller D. & Coan, M.E. Recent developments in elucidating tick vector relationships for anaplasmosis and equi (1995).
- Voigt, W.P., Young, A.S., Mwaura, S.N., Nyaga, S.G., Njihia, G.M., Mwakima, F.N. & Morzaria, S.P. In vitro feeding of instars of the ixodid tick Amblyomma variegatum on skin membranes and its application to the transmission of Theileria mutans and Cowdria ruminatum. Parasitology, 107, 257-63 (1993).
- Waladde, S.M., Young., A.S., Mwaura, S.N., Njihia, G.N & Mwakima, F.N. Transmission of Theileria parva to cattle by Rhipicephalus appendiculatus adults fed on nymphae in vitro on infected blood through an artificial Parasitology, 107, 249-256 (1993).
- Waladde, S.M., Young., A.S., Mwaura, S.N., Njihia, G.N & Mwakima, F.N. Optimization of the in vitro feeding of Rhipicephalus appendiculatus nymphae for thetransmission of Theileria parva. Parasitology, 111, 463-468 (1995).
- Waladde,. Young, A.S., Morzaria, A.S. Artificial feeding of ixodid ticks. Parasitology Today, 12, 272-278 (1996).

This work is licensed under a Creative Commons Attribution 4.0 International License.





