How to Cite | Publication History | PlumX Article Matrix
Ravichandra P., Annapurna Jetty*, Johny Joseph and A. Gangagni Rao
Biochemical and Environmental Engineering Centre, Indian Institute of Chemical Technology, Tarnaka, Hyderabad - 500 007 India
ABSTRACT: Continuous production of Oxytetracycline was carried out in a multiphase fluidized bed bioreactor with 30% (w/v) calcium alginate cross linked with glutaraldehyde immobilized cells of Streptomyces varsoviensis. At 1 vvm (unit volume of air per unit volume of the reactor per minute) of air flow rate maximum amount of antibiotic was produced in the reactor. In order to verify the oxygen transfer efficiency in STR and FBR, oxygen mass transfer coefficient was determined by using dynamic method at the end of steady state operation of the reactor after achieving highest antibiotic concentration. The oxygen mass transfer coefficient (kLa) obtained in fluidized bed bioreactor (FBR) was 35.7 1/hr whereas in Stirred tank reactor (STR) it was 27.9 1/hr under similar conditions. The results revealed that FBR could be operated more economically than STR because of 28% of higher oxygen mass transfer than STR for the production of antibiotics due to the higher oxygen transfer efficiency.
KEYWORDS: Dynamic Gassing out Method; FBR; Immobilization; Oxytetracycline; STR; Volumetric Oxygen Mass Transfer Coefficient (kLa)
| Copy the following to cite this article: Ravichandra P., Jetty A, Joseph J, A. Rao G. Oxygen Transfer Rate in Continuous Multi Phase Fluidized Bed Reactor with Immobilized Cells of Streptomyces varsoviensis for Production of Oxytetracycline. Biosci Biotech Res Asia 2006;3(2a). |
| Copy the following to cite this URL: Ravichandra P., Jetty A, Joseph J, A. Rao G. Oxygen Transfer Rate in Continuous Multi Phase Fluidized Bed Reactor with Immobilized Cells of Streptomyces varsoviensis for Production of Oxytetracycline. Biosci Biotech Res Asia 2006;3(2a). Available from: https://bit.ly/31V5ORz |
Introduction
Inadequate supply of oxygen is one of the major problems in industrial production of antibiotics. Efficient oxygen transfer is crucial especially for systems containing high biomass loading¹. In antibiotic production oxygen demand is the key parameter and hence oxygen supply plays a vital role in fermentation systems. It is difficult to meet oxygen demand especially in viscous fermentation broth. Special fermenter designs are required to enhance the rate of oxygen transfer in fermentation systems involving high oxygen demand (such as hydrocarbon fermentation). The design of a fermenter for optimum oxygen transfer requires a thorough understanding of the transfer and utilization of oxygen in microbial systems2. The dissolved oxygen (DO) concentration in a fermentation broth has a profound effect on theperformance of aerobic fermentation systems. The problem solving approach usually involves improvements in design of the bioreactor, agitator and sparger, as well as the use of oxygen –enriched air³.
The rate of oxygen mass transfer ultimately would limit the aerobic reactor performance. Oxygen transfer to the fermentation broth can limit both the extent and rate of cell growth. Mass transfer limitations to microbial flocs and immobilized cells can result in reduced reaction rates and inefficient conversion. Gas-liquid mass transfer is often rate limiting for gases that are sparingly soluble in the broth, such as oxygen in production media4.
Fluidized bed bioreactor5 is the most advanced reactor for fermentation process. It has better features when compared to Batch reactor and CSTR. The salient features are less pressure drop, uniform mixing, easy control and good aeration. The phases involved are gas, liquid and solid. The solid phase comprises of immobilized cells, in which biochemical reactions occur. The culture medium necessary for cell growth and maintenance constitutes the liquid phase. Gas phase is the supply of oxygen for respiration of the microbes and effective metabolite production. Earlier authors indicated enhancement of oxygen transfer with immobilized cells in a fluidized bed bioreactor6 and CSTR.7, 8
Streptomyces varsoviensis is an aerobic microorganism that is sensitive to dissolved oxygen changes. Below 30% saturation, the productivity diminishes9. In the absence of dissolved oxygen, the cells starve. According to earlier authors the cells are much more sensitive to oxygen starvation during the growth phase than during the production phase10. This indicates that lack of oxygen mainly influences the basic metabolisms rather than biosynthesis of antibiotics. During the growth and production phases, dissolved oxygen is necessary. Dissolved oxygen depends on the specific interfacial area of the bubbles in submerged cultures, which is a function of the property of the medium (viscosity, Coalescence-promoting/repressing properties). Oxygen mass transfer is a function of process cost. Hence, estimation of the oxygen mass transfer coefficient in continuous bio-reactor for the production of antibiotics is essential for the design and development of a bioreactor for efficient and economical production of antibiotics11 Various methods available for estimating volumetric oxygen mass transfer coefficient in aerobic processes are Dynamic gassing out, Oxygen balance, Sodium sulphite oxidation, Linear growth of strict aerobe, Bio-oxidation of catechol, Enzymatic oxidation of glucose, etc.12
The present study aimed at determination of volumetric oxygen mass transfer coefficient (kLa) using dynamic gassing out method in a continuous multi phase fluidized bed bioreactor with immobilized cells of Streptomyces varsoviensis for the production of Oxytetracycline and comparison of same with STR under similar conditions.
Materials and Methods
Microorganism and Culture Conditions
In the present study, the strain Streptomyces varsoviensis, NCIB-9522, MTCC-1537 was obtained from Institute of Microbial Technology, Chandigarh, India. It was maintained on Agar slopes using Actinomycetes Agar Medium (Hi Media Bombay). The growth medium contained (g l-1) of Glucose 4, Yeast extract 4, Malt extract 10, CaCO3 2, Agar 20, and distilled water 1 l, at pH 7, Temperature 28°C and subcultured at monthly intervals. Five ml of sterile distilled water was added to a well-sporulated slant (7-day old) and spores removed by scrapping. The spore suspension prepared was then added to a 250 ml Erlenmeyer flask containing 45 ml of growth medium incubated on a rotary shaker at 170 rpm at 28°C. After 72 hours of incubation, the culture broth was centrifuged, the cell pellet washed with sterile 0.9% NaCl solution. This cell suspension was used as inoculum for further experiments and also for the immobilization of whole cells.
Immobilization of Whole Cells
Cells were immobilized using Calcium alginate cross-linked with glutaraldehyde entrapment method13. 3% sodium alginate, 100µl glutaraldehyde was mixed with 0.06% cells on dry cell weight basis (DCW) (w/v) and mixed well, to get uniform suspension, then this solution was dropped into 0.2 M CaCl2 solution using peristaltic pump (Watson and Marlow. U.K.) through a cut micropipette tip or orifice to get an average beads of 2-2.5 mm. The beads then formed were cured for 24 hrs by incubating in 0.2 M CaCl2 solution and washed twice with sterile saline (0.9% NaCl w/v solution) and stored in saline solution at 4°C for further use. All these steps are carried out under aseptic conditions.
Operation of the STR and FBR
Experiments were conducted in batch STR (B. Braun Biostat, Germany) and FBR for the production of Oxytetracycline using calcium alginate immobilized cells of Streptomyces varsoviensis cross-linked with glutaraldehyde (Fig.-1). The composition of production medium consists of (g l-1) Glucose 30, Corn steep liquor 9, CaCO3 2, Ammonium sulfate 3.5, MgSO47H2O 0.1, CaCl2 0.005, Water 1 l at pH-7. The production medium was sterilized in an autoclave at 15lbs pressure and at 120°C except glucose. Glucose (30 g/l) sterilized separately and added to the production medium. The reactor and accessories were sterilized separately. The working volume of the both reactors were 1 liter and filled with 30% (v/v) of immobilized beads and the average size of the beads were in the range of 2-2.5 mm. Continuous sterile air was supplied through air filters (watman 50 µ) at the rate of 1vvm (unit volume of air per unit volume of the reactor per minute).
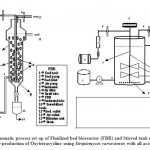 |
Figure 1: Schematic process set up of Fluidized bed bioreactor (FBR) |
The conical bottom of the FBR space was (where the draft tube is dipped) filled with autoclavable steel beads of 2mm diameter for distribution of air bubbles. During the operation of the reactor, it was observed that due to the large difference in relative density of calcium alginate beads and steels beads, the collisions of both the beads did not occur in the reactor, which reduced the stress on the calcium alginate beads. Feed was continuously supplied using peristaltic pumps (Watson and Marlow, U.K.) and for product collection.
Initially for the first four days, the FBR was operated in batch mode for the microorganism growth. From the 5th day onwards, the reactor was operated continuously by feeding substrate having the concentration of 15 g l-1 at the rate of 0.5 lit day-1. After achieving steady state in terms of antibiotic production during 8th to 11th day, the flow rate of media was increased to 0.6 lit day-1 and continued its operation till 15th day. Steady state was observed during this period and accordingly the flow rate was further increased to 0.7 lit day-1. At this feeding rate the antibiotic production dropped gradually and on 21st day reactor operation was discontinued. On 21st day, when the reactor was about to be discontinued, experiment for determining the kLa was carried out. Continuous Stirred tank reactor (STR) was also operated under similar conditions as of FBR. In the case of STR, impeller was provided for mixing with a speed of 300 rpm.
Analytical Methods
Samples were drawn in eppendorf tubes everyday under sterile conditions for antibiotic assay, carbohydrate estimation and pH. Oxytetracycline concentration was determined by plate diffusion assay using E.coli as test organism along with standard oxytetracycline disc (10mg, Himedia) in every plate14. Carbohydrate was estimated by Anthrone method15. The volumetric mass transfer coefficient (kL a) was determined using the dynamic method16 by measuring the dissolved oxygen concentration using B. Braun Biostat (Biotech International, Germany).
Theory for the determination of kLa by Dynamic method of gassing out16.
This method is probably the simplest one since it requires only a dissolved oxygen probe. It is mainly based upon the dynamic oxygen balance in a batch culture, which has the following form.
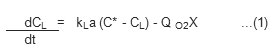
Where Q O2 is the rate of oxygen consumption per unit mass of cells (mm O2g –1h –1) Rearranging equation (1) yields.
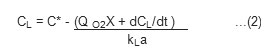
The air supply is turned off at a certain time during fermentation and the variation of CL with time is followed with the aid of a DO probe. Since the term kLa (C* – CL) becomes zero when air is turned off, the CL value decreases linearly with time according to equation (1). The slope of the CL versus time curve yields a value for Q O2X. The air is then turned on and the increase in DO with time is followed. Having determined the Q O2X value, CL is plotted against (Q O2X + dCL /dt). The slope of this plot is equal to the reciprocal of kLa.
Results and Discussion
Determination of kLa Values for STR
Substrate and immobilized cells were transferred to STR. Air was bubbled continuously to the reactor and antibiotic concentration was monitored regularly. On 4th day, it was observed that antibiotic concentration reached a value of 0.54 mg l-1. Therefore, continuous feeding of the substrate was started on 4th day and operated till 21st day. The antibiotic production of Oxytetracycline (OT) during the operation of the reactor was plotted and shown in Fig. -2. It could be observed from the figure that from16th day onwards, antibiotic activity started declining. The decline activity was observed till 21st day and experiment was terminated at this juncture. On the same day experiment was carried out for the determination of kLa by dynamic method. In STR the dissolved oxygen was measured for every 30 seconds up to 7.5 minutes. The dissolved oxygen (DO) concentration was stable at 3.2 mg l-1.
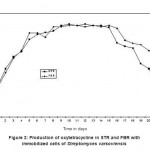 |
Figure 2: Production of oxytetracycline in STR and FBR with immobilized cells of Streptomyces varsoviensis |
Air supply was stopped at this condition (7.5th minutes) and decrease in DO is recorded until 25th minute. The DO concentration decreased from 3.2 to 0.8 mg l-1 during this period. Again air supply was started to the reactor at this juncture (25th minute) and DO was recorded. It was observed that DO recovered to 3.2 mg l-1 at 45th minute and remained stable further. The results were plotted and shown in a Fig. 3. Specific rate of oxygen consumption over a volume of liquid (QO2X) was determined by measuring slope of the curve during the absence of air supply from this graph. The rate of increase in DO (dCL/dt) was determined at four points when the air supply was resumed on 25th minute. Another plot was drawn (Fig. 4) between (QO2X+dCL/dt) Vs DO concentration. The slope of this graph was the reciprocal of the volumetric mass transfer coefficient. The kLa value for the STR was found to be 27.90 hr-1.
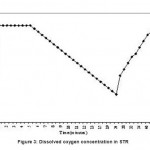 |
Figure 3: Dissolved oxygen concentration in STR |
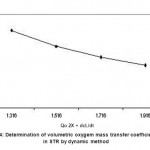 |
Figure 4: Determination of volumetric oxygem mass transfer coefficient (kLa) in STR by dynamic method |
Determination of kLa values for FBR
Substrate and immobilized cells are transferred to FBR. Air was bubbled continuously to the reactor contents and antibiotic concentration was monitored regularly. On 4th day, it was observed that antibiotic concentration reached a value of 0.62 mg l-1 (figure 2), which showed that cells started exhibiting activity by producing antibiotic as a secondary metabolite. Therefore, continuous feeding of the substrate was started on 5th day. The reactor was further operated for 17 days and it was observed that on 16th day, antibiotic activity started declining and therefore on 21st day experiments were terminated. On the same day experiment was carried out for the determination of kLa by dynamic method. In FBR the dissolved oxygen was measured for every 30 seconds up to 7 minutes. The dissolved oxygen (DO) concentration was stable at 3.5 mg l-1. Air supply was stopped at this condition (7th minute) and decrease in DO was recorded until 32nd minute. The DO concentration decreased from 3.5 to 1.5 mg l-1 during this period. Again air supply was started to the reactor from 32nd minute and DO recovered to the original stable value of 3.5 mg l-1 at 50th minute and remained stable further.
The results were plotted and shown in a Fig. -5. Specific rate of oxygen consumption over a volume of liquid (QO2X) was determined by measuring slope of the curve during the absence of air supply from this graph. The rate of increase in DO (dCL/dt) was determined at four points when the air supply was resumed at 32nd minute. Another plot was drawn (Fig. 6) between (QO2X+dCL/dt) and DO concentration. The slope of this graph was the reciprocal of the volumetric mass transfer coefficient. The kLa value for the FBR was found to be 35.7 hr –1. Oxygen transfer coefficient (kLa) studies reflect that confined cell fermentation maintains a much more favorable system, as the gas–liquid mass transfer properties of the culture become enhanced, possibly because of restructuring of the filamentous morphology of the cells.
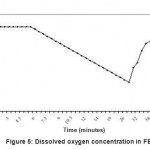 |
Figure 5: Dissolved oxygen concentration in FBR |
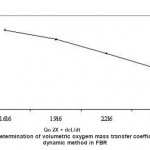 |
Figure 6: Determination of volumetric oxygem mass transfer coefficient (kLa) by dynamic method in FBR |
Comparison of kLa Values for STR and FBR
The kLa values which were determined for both STR and FBR are 27.90 hr –1 and 35.7 hr-1 respectively. The more the kLa value, the better the performance of the reactor in terms of oxygen mass transfer. Assessment of the real advantages of the immobilized process demands a rigorous study of the limitations to mass transfer of the reagents, especially oxygen17, 18. Due to high fluidization velocity created in FBR through the air, the kLa value was high in FBR when compared to STR. This showed the high performance of FBR in terms of high oxygen mass transfer rate. In an earlier studied for the production of cephalosporin, a comparison was made between STR and multi phase air lift reactor with immobilized cells and the results of the study reveals that 60% of energy can be saved in case ALR19 and FBR20 with respect to STR. It could be noted that oxygen mass transfer coefficient in fluidized bed bioreactor was 28% higher than STR. It could be derived from these results that FBR could be operated more economically than STR for the production of antibiotics21,22. Use of immobilized cells in fluidized bed reactor will increase the oxygen mass transfer rate7,8,23.
Conclusions
High volumetric oxygen mass transfer coefficient in fluidized bed bioreactor represented excellent performance in terms of good aeration and antibiotic production. This information is useful for design and economical operation of multi phase fluidized bed bioreactor with immobilized cells of Streptomyces varsoviensis for the production of Oxy tetracycline.
Acknowledgments
The Authors are grateful to Dr.J.S.Yadav, Director, IICT, for his encouragement and thankful to DST (SERC), Govt. of India for funding the project.
References
- Bailey, J.E., Ollis, D.F., Biochemical Engineering Fundamentals. 2nd, Mc Graw-Hill, New York, 635 (1986).
- Duran, P.M, Bioprocess Engineering Principles, Xth ed., Academic Press 198 (2003).
- Moo-Young, M., Blanch. H.W., Design of biochemical reactors, Biochem. Eng. 19, 1 (1981).
- Bailey, J and D.F. Ollis, Biochemical Engineering Fundamentals, second ed., McGraw Hill International Editions 606 (1986).
- Godia, F., Sola, C., Fluidized bed bioreactors. Prog. 11, 479-497 (1995)
- Chevalier, P., Dela Noue, J., Enzyme Micro. , 10, 19 (1988).
- Swaroopa Rani A., Annopurna Jetty, and Ramakrishna S. V., Biochem. Eng. Q. 17, 119 (2003).
- Annapurna Jetty, A. Gangagni Rao, B. Sarva Rao, G. Madhavi, and S. V. Ramakrishna, Biochem. Eng. Q. 19, 179 (2005).
- Vardar, F., Lilly, M.D., Bioeng. 24, 1711 (1982).
- Larsson, G., Enfors, S.O., Micro. Biotech., 21, 228 (1985).
- Murat Elibola, Ferda Mavituna, Eng J., 3, 1 (1999).
- Kenny Ortiz-Ochoa, Steven D. Doig, John M. Ward b, Frank Baganz, Eng. J., 25, 63 (2005).
- Marek, P.J., Kierstan, M., Coughlan, M.P., Immobilized cells and Enzymes. A practical approaches UK, Ireland press limited 43 (1985).
- Bauer, A.W., Kirby, W.M., American J. Clin. Pathol., 45, 491 (1996).
- Loewus, F.A., Chem., 24, 219 (1952).
- Kargi and Murray moo young, Comprehensive biotechnology, Vol 2, Design and operation of bioreactors, 21, pergamon press, Canada 21 (1985).
- Xuemei Li, Xiao Dong Chena, Naixing Chen Eng. J., 17, 65 (2004).
- Araujo, M.L.G.C., R.C. Giordano, C.O., Hokka, Bioeng., 63, 593 (1999).
- Pradeep Srivastava and Subir Kundu, Biochem., 34, 329 (1999).
- Rodr´ýguez Porcel, F.M., J.L. Casas L´opez, A. S´anchez P´erez ,J.M. Fern´andez Scheugerl, K, J. Biotech. 13, 251 (1990).
- Elma zbek, Sevgi Gayik, Biochem., 36, 729 (2001).
- Sevilla , Y. Chisti, Eng. J. 26, 39 (2005).
- Swaroopa Rani, Annapurna Jetty and S.V. Ramakrishna, J. Biotech., 3, 394 (2004).
Abbreviations
FBR → Fluidized bed bioreactor
STR → Stirred tank reactor
DO → Dissolved oxygen
OT → Oxytetracycline
ALR → Air lift reactor
Nomenclature
kLa → Volumetric oxygen transfer coefficient, hr-1
CL → Saturated dissolved oxygen concentration in liquid, mg l-l
C* → Equilibrium dissolved oxygen concentration in liquid, mg l-l
X → Biomass concentration in liquid, mg l-l dCL/dt → Change in DO concentration in liquid with time, mg l-l hr-l
QO2 → Specific rate of oxygen consumption m M O2 g-l

This work is licensed under a Creative Commons Attribution 4.0 International License.





