How to Cite | Publication History | PlumX Article Matrix
Flavanoid From the Roots of Citrus Sinensis
Javed Intekhab, N. U. Siddiqui and Mohammad Aslam*
Natural Products Research Division, Post Graduate Department of Chemistry, G.F.College (Rohailkhand University), Shahjahanpur India.
ABSTRACT:
From the ethyl acetate extract of the roots of Citrus sinensis by eluting with chloroform-methanol (7:1) a flavanoid characterized as 5-hydroxy-2-(42 -hydroxyphenyl) -2", 2",-dimethyl-2, 3-dihydro-pyrano-chromen-4-one was isolated. The compound was characterized by elemental analysis, U.V., I.R., N.M.R and mass spectral studies.
KEYWORDS: Flavanoids; Citrus sinensis
Download this article as:| Copy the following to cite this article: Intekhab J, Siddiqui N. U, Aslam M. Spectrophotometric Estimation of Tenofovir in Pharmaceutical Formulations. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Intekhab J, Siddiqui N. U, Aslam M. Spectrophotometric Estimation of Tenofovir in Pharmaceutical Formulations. Biosci Biotechnol Res Asia 2008;5(1) Available from: https://www.biotech-asia.org/?p=6993 |
Introduction
Citrus is one of the genus of family Rutaceae considered to be important folk medicine for the treatment of number of diseases1. According to CSIRO (Commonwealth Scientific and Industrial Research Organization) 2 Citrus fruit can reduce the risk of mouth, larynx and stomach cancer. The report based on 48 international studies on the benefits of citrus fruits, found that citrus could reduce risk of cardiovascular diseases, obesity and diabetes.
Citrus fruits contain hundreds of phytochemical and there is increasing evidence that these substances contribute to optimal health and may protect against some of the chronic diseases such as cancer and cardio vascular disease, degenerative eye and general damage caused by aging.
More than 170 different phytochemical and about 60 flavonoids, found in different citrus species, have been shown to exhibit anti-inflammatory, anti-tumour, blood clot inhibiting properties and anti-oxidant effects3-4. A pharmacological study showed that citrus juice (grape and orange) reduces the risk of forming calcium oxalate stones (kidney stones). A recent study by the WHO also found convincing evidence of positive effects from the dietary intake of Citrus fruits on cardiovascular disease5-8.
Various species of the genus Citrus are valued for their medicinal importance. Citrus sinensis is one of the species of this genus, abundantly available in India, Pakistan and Bangladesh, widely used for the treatment of various ailments.
The genus Citrus is a rich source of flavanoids, carotenoids, coumarins, monoterpenes, phenolic acids, sterols, hydrocarbons, glucosides, phospholipids, fatty acids, psoralens and limonoids9-25.
Result and Dicussion
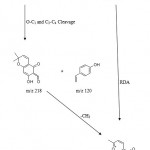
The compound was obtained as yellow powder (mp. 140-142 0C) by eluting column with chloroform and methanol (7:1) from the EtOAc extract. Its molecular ion peak obtained in its mass spectra at m/z 338 corresponds to the molecular formula C20H18O5. The retro Diels-Alder process from molecular ion was an important decomposition mode found in the mass spectra of flavone. The ion at m/z 218 was found from the RDA fragment-containing ring B and the small peak at 120 was also due to this fragmentation, suggested the presence of dimethyl pyran ring on the A-ring. The I.R spectrum of the compound exhibited absorption bands at 3442 cm-1 (OH group), 1616 cm-1 (conjugated carbonyl group) and 1263, 1155 (ether) functionalities.
The 1H NMR studies showed it to be flavonoid with 1, 4 disubstituted aromatic ring along with a dimethyl pyran ring attached to ring A. Two two proton doublets observed in its 1H NMR spectrum at δ7.34 (J=8.4 Hz) and δ 6.89(J=8.4 Hz) were clearly assignable to ring B proton at H-2′, H-6′ and H-3′, H-5′ respectively. Two double doublet appeared at δ 2.75(J=17.0, 3.0 Hz) and 3.0(J=17.0, 12.8 Hz) were assigned to two H-3 proton of pyron ring. Another one proton double doublet observed at δ 5.34(J=13.0, 3.0 Hz) was assigned to H-2 proton of the pyron ring. The two doublets corresponding to one proton each appeared at δ 5.54 (J=10.0 Hz) and δ 6.67 (J=10.0Hz) were assigned to the protons H-3“ and H-4“ of pyran ring attached to ring A. The two-methyl group’s protons of pyran ring resonated as singlet at δ 1.45. Another one-proton singlet observed at δ 6.0 was assigned to ring A proton H-6 was in support of the attachment of dimethyl pyran ring to C-7 and C-8 positions of ring A.
The structure was further supported by its 13C NMR spectrum, which demonstrated a downfield signal at 190.2 clearly assignable to carbonyl carbon at C-4. The two downfield signals appeared at 158.2 and 156.6 were assigned to C-5 and C-4′ carbon atoms bearing hydroxyl group. The signals observed at 26.3 for two methyl groups were further in confirmative of dimethyl substitution in the molecule.
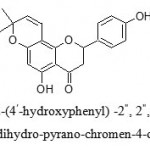
Thus structure of compound was established as 5-hydroxy-2-(4′-hydroxyphenyl) -2“, 2“,-dimethyl-2, 3-dihydro-pyrano-chromen-4-one. It is found to be similar to the previously reported compound Citflavanone, which was further supported by the formation of diacetate compound on acetylation.
5-hydroxy-2-(4′-hydroxyphenyl) -2“, 2“,-dimethyl-2, 3-dihydro-pyrano-chromen-4-one
Experimental
Ultra violet absorption spectrum was recorded on Perkin-Elmer Lambda Bio 20 UV spectrometer. I R spectroscopy was performed on Perkin-Elmer 1710 infrared fourier transformation spectrometer. NMR spectra were recorded on Bruker AVANCE DRX- 300(300 Hz). MS was recorded on JEOLSX 1021/DA-6000 mass spectrometer.
 |
Scheme 1
|
Plant material
The roots of Citrus sinensis were collected from the rural areas of district Shahjahanpur in the month of April and a specimen sample was preserved in the department of botany.
Extraction
Air dried roots of Citrus sinensis were first defatted with petrol (3lt x 5 times) and then soxheleted with ethyl acetate and methanol (3lt x 5 times each). The EtOAc extract was then evaporated under vacuum on rotatory evaporator below 50 0C temperature to yield a brownish mass (60 gm). The mass was then subjected to column chromatography. A well-stirred suspension of silica gel (100 -150 g in pet-ether 60-800) was poured into column (150 cm long and 50 mm in diameter). When the absorbent was well settled, the excess of petrol was allowed to pass through column. Slurry was made to this mass with silica gel in pet-ether and was digested to well stirred column. The column was successely eluted with the solvents and their mixtures of increasing polarity. Elution with CHCl3: MeOH (7:1) afforded a yellow powder.
Compound
Physical state : Yellow powder
M.P. : 140-142 0C
Rf : 5% MeOH in CHCl3 10.5 min.
UV (MeOH) λmax : 271, 362, nm
IR (KBr) νmax : 3442, 1616, 1263, 1155 cm-1
Mass spectra m/z : 338 [M] +, 323 [M-CH3] +, 218[M-120] +,
203[M-120-CH3] +, 161, 120, 83, 73
1H NMR (400 MHz, CDCl3) δ ppm : 12.30(1H, br s, 5-OH), 7.34(2H, d, J=8.4Hz,
H-2′, H-6′), 6.89 (2H, d, J=8.4Hz, H-3′, H-5′), 6.67 (1H, d, J=8.4Hz, H-4”), 6.43 (1H, s, 4′-OH), 6.00(1H, s, H-6), 5.54 (1H, d, J=10.0 Hz, H-3”), 5.34 (1H, dd, J=13.0, 3 Hz , H-2), 3.00 (1H, dd, J=17.0, 12.8 Hz, H-3), 2.75 (1H, dd, J=17.0, 3 Hz, H-3), 1.45 (6H, s, 5”, 6”- CH3)
13C NMR (100 MHz, CDCl3) δC, ppm: 126.3(C-6”), 26.3 (C-5”), 43 (C-3), 78.5 (C-
2), 78.2 (C-2”), 102.3 (C-8), 102.5 (C-10), 111.3 (C-5′), 115.1 (C-4”), 115.3 (C-3′), 127.1 (C-2′), 127.2 (C-3”), 127.8 (C-6′), 130.3 (C-1′), 158.2 (C-5), 156.6 (C-4′), 162.0 (C-9), 162.1 (C-7), 163.5 (C-6), 190.2 (C-4)
 |
Scheme 2
|
References
- Chopra, R.N., Nayar, S.L. and Chopra, I.C. (1956) Glossary of Indian medicinal plants, CSIR, New Delhi.
- W.H.O., Diet, Nutrition and Preservation of Chronic Disease, (2003) WHO/FAQ Expert Conclusion, Geneva
- Iinuma, M., Matsuura, S., Kurogochi, K. and Tanaka, T. (1980). Chem.Pharm.Bull, 3,717
- Yoshimizu, N., Otani, Y., Saikawa, Y., Kubota, T., Yoshida, M., Furukawa, T., Kumwi, K., Kameyama, K., Fujii, M., Yano, M., Sato, T., Ito, A. and Kitajima, M. (2004). Aliment Pharmacology Therapy, 20, 95.
- Yu, H., Robert, R.T. and Ho, C.T. (2004). Abstracts of Papers, 227th ACS National Meeting, Anaheim, CA, United States, March 28-April 1. AGFD-025
- Hertog, M.G.L., Feskens, E. J. M., Hollmann, P.C.H., Katan, M.B. and Kromhout, D. (1994), Natur. Conc. 22, 175
- Hertog, M.G.L., Feskens, E.J.M., Hollmann, P.C.H., Katan, M.B. and Kromhout, D. (1994), Lancet, 342, 1007
- Hirvonen, T.,Virtamo, J., Korhonen, P., Albanes, D. and Pietinen, P. (2000) Stroke, 31, 2013
- W.H.O., Diet, Nutrition and Preservation of Chronic Disease, (2003) WHO/FAQ Expert Conclusion, Genera
- Swift, L. J. (1952). J. Am. Chem. Soc. 74, 1099
- Gentili, B. and Horowitz, R.M. (1964). Phytochemistry, 20, 2313
- Maier, V.P. and Metzler, D.M. (1967). Phytochemistry, 6, 1127
- Nordby, H.E. and Nagy, S. (1969). Phytochemistry, 8, 2027
- Mayer, V.P. and Margileth, D.A. (1969). Phytochemistry, 8, 243
- Vander Cook,C.E., Price, R.L.and Guerrero, H.C.(1970).J.Agr.Food.Chem., 8,905
- Tatum, J.H. and Berry, R.E. and Jurd, L. (1970). Agric. Food Chem., 18, 500
- Lund, E. D., Richard, L. and Moshonas, M. G. (1970). Phytochemistry, 9, 2419
- Nordby, H.E. and Nagy, S. (1971). Phytochemistry, 10,615
- Stanley, W. L. and Jurd, L. (1971). Agric. Food chem., 19, 1106
- Nagy, S. and Nordby, H. E. (1971). Phytochemistry, 10, 2763
- Tatum, J.H. and Berry, R.E. (1972). Phytochemistry.11, 2283
- Hsu, A. C., Hasegawa, S., Maier,V.P. and Bennett, R.D. (1973), Phytochemistry,12,563
- Tatum, J.H. and Berry, R.E. (1977). Phytochemistry, 16, 1091
- Gray, A. I. and Waterman, P.G. (1978). Phytochemistry, 17, 845
- Chatterjee, P. and Chatterjee, A. (1988).Phytochemistry.27 (3), 946
- Lin, N., Sato, T., Tkayama, Y., Mimaki, Y., Sashida, Y., Yano, M. and Ito, A. (2003). Biochem. Pharmacol. 65, 2065

This work is licensed under a Creative Commons Attribution 4.0 International License.





