How to Cite | Publication History | PlumX Article Matrix
Immobilization of polyphenol oxidase purified from Igdir Apricot on bardakci-clay
Halit Demir1, aycan Gur1, adnan yilidz1 and tugba Gur2
1Science and Arts Faculty, Department of Chemistry, Yuzuncu Yil University Van-Turkey.
2Education Faculty, Department of Science, Yuzuncu Yil University Van-Turkey.
ABSTRACT: It is known that immobilized enzymes more useful and advantageous than free enzymes. In this work, polyphenol oxidase purifed by affinity chromatography from Igdir apricot was immobilized onto natural bardakci-clay by physical adsorption method. The properties of the immobilized enzyme were compared to the free enzyme. The PPO activity of immobilized clay was determined, and then the effects of reaction optimum temperature, thermostability, optimum pH, ionic effect and kinetic parameters were investigated. Catechol was used as substrate, the activities of immobilized and free polyphenol oxidase were determined in the reaction mixture containing substrate catechol. The results obtained from experiments indicated that physical adsorption is favourable for attachment of enzyme onto bardakci-clay.
KEYWORDS: Polyphenol oxidase; clay; purification; immobilization
Download this article as:| Copy the following to cite this article: Demir H, Aycan Gur A, Yilidz A, Gur T. Immobilization of polyphenol oxidase purified from Igdir Apricot on bardakci-clay. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Demir H, Aycan Gur A, Yilidz A, Gur T. Immobilization of polyphenol oxidase purified from Igdir Apricot on bardakci-clay. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6513 |
Introduction
In literature it is seen that use of the immobilized enzymes have proved to be more advantageous than free enzymes[1]. Immobilization of enzyme through physical methods is still most commonly used because it is the easiest to perform and the least expensive. In addition, both organic and inorganic materials such as porous glass, silica gels, hydro gels, and cellulose are used for preparation of immobilized enzymes[2,3]. Enzyme immobilization by physical adsorption has the benenefit of a wide applicability and may provide a practical convenience of simple regeneration of support by removing the deactivated enzyme and reloading the support with fresh batch of active catalyst[4]. Purfied PPO from diffrent sources have been succesfully used for supports[5].
In this study, polyphenol oxidase purified from Igdır apricot was immobilized on bardakcı-clay and physicochemically characterized. And in the light of experimental results obtaining values were evalued by means of kinetic parameters.
Experimental
preparation of materials
Catechol, benzoic acid, sodium azide, HCI, ascorbic acid, sodium phosphate were obtained from Sigma Chemicals. All other chemicals and solvents used in this study were of analytical grade. Igdır apricot was obtained from the vegetable garden from Igdır-Turkey.
Extraction and purification of apricot PPO by affinity chromatography
To purify polyphenol oxidase enzyme obtained from Igdır apricot, phosphate buffer at 7.3 pH was used, necessary centrifuging and other processes were carried out and the homogenate to be applied to the column was prepared. The homogenate was applied to activated Sepharose 4B-Tyrosine-p-aminobenzoic asid affinity column. Activity showing fractions obtained from column, for quantitative protein analyze was performed at 595 nm with Coomassie blue method.
Immobilization of clay
1.5 g clay was taken and mixed with 60ml pure water, then 4 ml of clay-water mixing was taken to form 1ml enzyme solution. After the mixture was to be formed, it was shaked by vortex approximately one hour, and then it was centrifuged at 3000 rpm for 15 minute. Furthermore 5 ml phosphate buffer dispersed into filtrate and the mixing was centrifuged again at 3000 rpm, 15 minute and finally the clay-enzyme mixing was obtained. The immobilized enzyme was then dried in freeze drier.
Protein assay
Protein determination was done according to method described by Bradford. Bovine serum albumin was used as a standard[6].
Characterization of the immobilized polyphenol oxidase
% Immobilization = Total amount of protein in supernatant before immobilization
-total amount of protein in supernatant after immobilization X 100
total amount of protein in supernatant before immobilization
Effect of temperature on activity of PPO
Clay-enzyme mixture were reacted at different temperatures ( 20, 30, 40, 50, 60, 70, 80 and 90 oC) for 1 hour at 150 rpm in a water bath shaker.
Effect of pH on activity of PPO
Immobilized and free PPO activity were determined at various pH (4-8) values, using catechol as a substrate.
Thermal Stability
Immobilized and free polyphenol oxidase were kept during 10 days at 20 oC before the enzyme determination.
Kinetic of parameters
Vmax and Km values both free and immobilized polyphenol oxidase were determined by Lineweaver-Burk graphs.
Effect of ionic strength
Effect of ionic strength on immobilized and free polyphenol oxidase were investigated.
Results and Discussion
Enzymes are often immobilized onto solid support or various materials. Immobilized enzymes should process mechanical strength, microbial resistance, thermostability, chemical durability, chemical functionality, low cost, hydrophilicity, regenerability and a higt capacity of enzyme[7]. Polyphenol oxidase purified from apricots are important enzymes with respect to its availability and cost. Immobilized polyphenol oxidase has been successfully used for the treatment of wastewater by removing or transforming the toxic compounds of industrial procces[4]. It is shown that polyphenol oxidase can be immobilized on capillary membranes facilitating the conversion of phenols to corresponding o-quinones [8]. In another study, the immobilized potato PPO has been successfully immobilized on Celite 545. The stability of immobilized potato PPO has been investigated against various forms of denaturants; pH, heat, urea, detergents and water-miscible organic solvents[5]. The diatomite carries have been used as a support for the immobilization of enzymes[9,10]. Several investigators have been already reported the immobilization of potato PPO via adsorption on chitin, chitosan and Celite 545 supports [5,11,12]. In another work, Km values have been found 0.65 and 0.87 mM for the free and the immobilized enzyme and Vmax values have been found to be 1890 and 760 U mg-1 for the free and the immobilized enzyme, respectively[22]. Immobized PPO was used for transform toxic compounds in industrial proccesses, and to control pollution in water[23-25]. In our study the immobilized enzyme gave a Km value of 47,61 mM as compared with 43,47 mM for the free enzyme. Vmax values were found to be 0.0087 (IU/mL. min) and 0.2636 (IU/mL. min) for the free and immobilized enzyme, respectively. The amount of protein before and after the immobilization was determined by using the method of Coomassie brilliant blue assay procedure using bovine serum albumin as standard[6]. Using the Bradford method, the protein content in free PPO before immobilization was found to be as 0.9 mg. After the immobilization, protein content in supernatant was found to be 0.04 mg. From the calculation, it was determined that approximately 95.5 % of protein in supernatant from PPO was immobilized onto the support.
Effect of pH
Many earlier workers have also reported the pH- dependent adsorption of polyphenol oxidase on various other supports[12]. Both soluble and Celite bound potato preparations exhibited pH-optima at pH 6.0[4]. However, there are not difference in pH-optima between pH 5 and pH 6 for the immobilized potato PPO. This worker has been done in agreement with other earlier published work[9,13]. Polyphenol oxidase from potato are copper containing proteins[14]. The optimum pH for PPO from other studies has been reported [15-17]. Furthermore there have been found some values for raspberry as 8 , 5.5 [18], and for Allium sp as 7.5 [19], and for amasya apple as 7, respectively [20]. It was obtained that the optimum pH have been 6.5 for the free and 7.0 for the immobilized enzyme, respectively[22]. The activity of PPO was determined at different pH values during incubations at 25 oC in sodium phosphate buffer. The optimum pH for PPO activity both free and immobilized were found as 7 and 6 in catechol, respectively (fig. 1 and 2). The optimum pH of immobilized mushroom PPO was given as 7 and its activity was found similar to our findings[26].
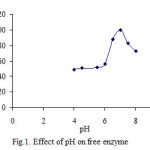 |
Figure 1
|
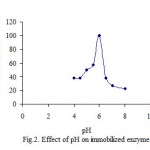 |
Figure 2
|
Effect of temperature
Several investigators have been also done an alternation in temperature-optima, of fig tree latex ficin immobilized on Celite, from 60 to 80 oC[9]. Its results findings demonstrated that the Celite bound polyphenol oxidase has been remarkably more stable to heat inactivation[4,5]. Its that the optimum reaction temperature for the free enzyme has been found 40 °C and for the immobilized enzyme 45 °C[22]. The effect of reaction temperature using free PPO and clay-immobilized PPO on the activity was shown (fig.3 and 4). The results show that the highest percentage of activity of free and immobilized PPO was 40 and 60 oC respectively. Its seems that clay might protecting the enzyme against denaturation at higher temperature.
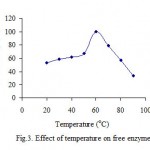 |
Figure 3
|
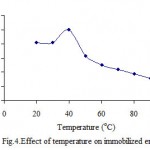 |
Figure 4
|
Effect of ionic strength
Effect of ionic strength on polyphenol oxidase immobilized and free were investigated. It was shown that direct immobilization of polyphenol oxidase on Celite 545 from ammonium sulphate fractionated proteins potato[4]. Several investigators have been already shown the immobilization of potato PPO via adsorption on chitin, chitosan and Celite 545 supports resulted in the stabilization of PPO activity against water-miscible organic solvents[5,11,12]. The results show that the highest percentage of the activity of free and immobilized PPO were at 0,08 and 0,1 M, respectively (fig.5 and 6).
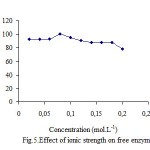 |
Figure 5
|
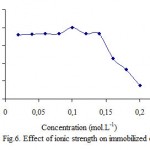 |
Figure 6
|
Effect of time
Bardakcı-clay, which is obtained from region of Van-Turkey, is an economical support for enzyme immobilization. For both immobilized and free ezyme PPO retained during 10 days, at 20 oC. The results indicated that immobilized enzme was more stable than free enzyme (fig. 7 and 8).
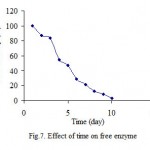 |
Figure 7
|
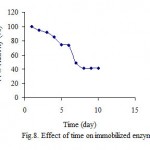 |
Figure 8
|
Conclusions
The optimum pH for PPO activity both free and immobilized were found as 7 and 6 in catechol, respectively. When both immobilized and free ezyme PPO retained during 10 days and at 20 oC, it was seen that the activity of PPO reduced. The results show that the highest percentage of activity of free and immobilized PPO were 40 and 60 oC respectively. The results indicated that immobilized enzyme was more stable than free enzyme. And consequently, it can say that physical adsorption is suitable for the attachment of enzyme onto bardakcı-clay as support.
References
- Duran MA, Rosa AD’Annibale, Gianfreda L, Enzyme Microb. Technol 2007; 31:907.
- Baileey JE, Ollis DF. Applied Enzm. Catalysis Mc Graw-Hill International, 1986: 180
- Kennedy JF, M.Melo EH, Chem.Eng.Prog 1990:81.
- Khan AA, Akhtar S, Husain Q, J. of Molcular Catalysis B: Enzymatic 2006; 40 58.
- Khan AA, Akhtar S, Husain Q, Procces Biochemistry 2005; 40:2379.
- Bradford MM, Anal. Biochem1976;72:248.
- Burns RG, Soil Science Society of America 1986: 439
- Edwards W, Bownes R, Leukes WD, Jacobs EP, Sanderson RD, Rose PD, Burton SG Enzyme Microb Tech 1999:209.
- Anderson WA, Pay P, Legge RL, Moo-Young M, J. Chem. Technol. Biotechnol 1990; 47:93.
- Fadyloğlu S, Nahrung-Food 2001; 45:143.
- Batra R, Gupta MN, Biotechnol. Lett. 1994;16:1059.
- Batra R, Gupta MN, Biotechnol. Appl. Biochem. 1994;19:209.
- Soysal C, Söylemez, Z Nahrung-Food 2004;48:5.
- Partington JC, Bowell GP, Phytochemistry 1996; 42 1499.
- Demir H, Bull. Pure Appl.Sci.Chem. 2004;23:1.
- Das RJ, Bhat GS, Gowde RL, J.Agric. Food Chem. 1997;45:2041.
- Jang YM, Food Chem. 1999;66:75.
- Gonzalez EM, Ancos BD, Cano MP, J.Agric. Food Chem. 1999;47:4068.
- Arslan O, Temur A, Tozlu I, J.Agric. Food Chem. 1997;45:2861.
- Oktay M, Küfrevioglu I, Kocaçalişkan I, Sakiroglu H, J.Agric. Food Chem. 1995;60:494.
- Yang CP, Fujita S, AhrafuzzamanMD, Nakamura N, Hayashi N, J.Agric. Food Chem. 2000;48:27632.
- Yakup Arca M, Polymer International 2000:49: 775.
- Payne GF, Sun WQ, Sohrabi A, Biotech. Bioeng. 1992;40:1011.
- Wada S, Ichikawa H, Tatsumi K, Biotech. Bioeng. 1993;42:854.
- Burton SG, Boshohh A, Edwards W, Rose PD, J.Molecular Catalysis B:Enzymatic 1998;5:411.
- Estrada P, Sanchez-Muniz R, Acebal C, Arche R, Castillon M, Biotechnol. Appl. Biochem. 1991;14:12.

This work is licensed under a Creative Commons Attribution 4.0 International License.





