How to Cite | Publication History | PlumX Article Matrix
In vitro degradation of chicken feather paper by Bacillus subtilis
Sreenivasulu1*, R. B. Choudary1, V. Chandra Prakash1 And N. M. R. Bhargava2
1K.L. College of Engineering, Vaddeswaram - 522 502, Guntur District (India) 2College of Engineering, Andhra University, Visakhapatnam - 530 003 (India)
ABSTRACT: Chicken feather is generated in large quantities as a waste product of poultry processing industry. Morphologically chicken feather fiber (CFF) is akin to cotton fiber. Researchers developed technologies to produce CFF paper. CFF undergoes various chemical treatments during production of paper which might affect its bio degradability. The paper presents the results of in vitro bio degradability of CFF paper by Bacillus subtilis. Changes in pH of the medium, release of disulphides, soluble proteins, amino acids and rate of keratinase activity of the culture filtrate were estimated during the degradation process. SDS-PAGE results show that in vitro degradation reached saturation in four weeks. The enzyme keratinase produced by the Bacillus Subtilis was found to be responsible for the effective biodegradation of CFF paper. The results confirm that CFF paper degrades rapidly and is eco friendly.
KEYWORDS: Feather; keratin; handmade paper; biodegradability; pollution; keratinase; Bacillus subtilis
Download this article as:| Copy the following to cite this article: Sreenivasulu K, Choudary R. B, Prakash V. C, Bhargava N. M. R. In vitro degradation of chicken feather paper by Bacillus subtilis. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Sreenivasulu K, Choudary R. B, Prakash V. C, Bhargava N. M. R. In vitro degradation of chicken feather paper by Bacillus subtilis. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6756 |
Introduction
The poultry industry in India produces nearly one hundred thousand tonnes of feathers each year1 which is going almost as waste. Generation of chicken feathers in India is scattered due to small retail selling centers that are concentrated mostly in urban and sub-urban areas. Traditional feather disposal methods are unhygienic resulting in serious environmental pollution. Indiscriminate disposal of the feather waste in the municipal dust bins and in canals, which goes mostly unchecked, is resulting in ecological imbalance. Incineration and land filling are also limited due to heavy expenditure involved.
Feathers are composite strands of innumerable parallel microcrystalline fibers (barb) held together on a rigid central quill. Chemically, both barb and quill components are all keratin2. Feather barbs or fibers are said to have a structure that is of the honeycomb type with hexagonal cell structure, which is strong and light. The barbs range from 20 to 40 mm in length with diameter ranging from 6 to 8 µm.
Barb is more stable because it is oriented as a helices at the molecular level. In contrast, the quill exists in a less ordered b sheet conformation. This molecular ordering affects physical and chemical properties. Due to high degree of disulphide bonds, hydrophobic interactions and
H-bonds, keratin in its native state is not degradable by common proteolytic enzymes such as pepsin, trypsin and papain3 & 4. Keratinolytic micro organisms can grow on feather as it is an organic substrate providing Carbon, Nitrogen, Sulphur and energy5. Keratin is a fibrous protein with a molecular weight ranging from 59,000 to 65,000 and consists of polypeptide chains formed by the condensation of different amino acids6. The amino acid content of feathers is given in Table 1.
Table 1: Amino acid composition of feathers.
| Functional | Amino | Content |
| groups | acid | as % |
| mole | ||
| Negatively charged
acid |
Aspartic
|
5 |
|
acid |
Glutamic
|
7 |
| Positively charged | Arginine | 5 |
| Conformationally | Proline | 12 |
| special | ||
| Glycine | 11 | |
| Hydrophobic
ne |
Phenylalani
|
4 |
| Alanine | 4 | |
| Cystine | 7 | |
| Valine | 9 | |
| Isoleucine | 5 | |
| Leucine | 6 | |
| Tyrosine | 1 | |
| Hydrophilic | Threonine | 4 |
| Serine | 16 |
The high content of cystine makes the protein stable due to its a-amino and a-carbonyl groups, being able to form a network structure by joining adjacent polypeptides. Intermolecular forces such as Vanderwaals forces, disulfide, hydrogen, ionic, and hydrophobic bonds, due to the basic side groups contained in the above amino acids, stabilize the a-helix keratin structure. Keratin fiber is made up from a crystalline fiber phase and an amorphous protein matrix phase linked to each other. The crystalline phase consists of a-helical proteins braided into microfibrils, where as the protein matrix is anchored by means of H-bonds and the intermolecular interactions as mentioned earlier. The tight packing of the keratin chain in a helix or b sheet into a super coiled polypeptide chain resulted in mechanical stability and resistance to proteolysis7.
Keratinolytic microbes degrade proteinaceous keratin substrate. Research on biodegradation of keratin has been carried out with fungi and actinomycetes8 & 9. Noval and Nickerson worked on streptomyces fradiae and their results established that the degradation of keratin is not only due to extra cellular keratinase but is also accompanied by direct reduction proteolysis and finally by alkalization of the medium. The biochemical mechanism of keratin degradation by bacteria centers on three interlinked processes viz. denaturation of substrates, proteolysis and de-amination.
putrificus, B. subtilis, B. cereus and B. mesentericus are important bacteria involved in degradation of keratinaceous materials like wool, animal fibers, avian feathers etc. Choudary R.B., one of the authors of this paper, has developed a technology and prepared hand made paper using CFF10. The handmade CFF paper was prepared at Kumarappa National Handmade Paper Institute (KNHPI) situated at Ramsing Pura, Sanganer, Jaipur, Rajasthan. Figure 1a shows hand made CFF paper. Scanning Electron Micrograph (Fig. 1b) of CFF paper shows adequate interlocking of fibers. In vitro degradation of the hand made CFF paper using B.Subtilis has been carried out and the results were analyzed in this study.
Material and Methods
Microorganism
Bacillus Subtilis (NCIM-2724) procured from National Chemical Laboratory, Pune, India
Medium
Basal salt medium containing (g/L) K2HPO4 – 1.5, MgSO4.7H2O – 0.025, CaCl2 – 0.025, FeSO4.7H2O – 1.5, ZnSO4.7H2O – 0.005, pH – 6.5
Enriched medium
Basal salt + 5 g of CFF paper.
Substrate sterilization
Paper and medium were sterilized by placing in Autoclave at 121°C for 20 min at 15 psi pressure.
Inoculum
A loopful of 10 day old enriched culture was added to sterile basal salt medium and it is divided into 3 sets.
Keratin control: 2 g CFF paper + 100 ml medium
Bacterial control: 2 ml inoculum + 100 ml medium
Test samples: 2 g CFF paper +100 ml medium + 2 ml inoculum
All flasks were placed in incubator at 31 ± 1oC for 5 weeks in triplicates.
Analysis: Aliquots were removed at end of each incubation period through pre-weighed Whatman filter paper No 42. The culture filtrate was centrifuged at 4,000 rpm for 6 min and the supernatant was subjected to the following biochemical tests.
Determination of pH: Changes in alkalinity of culture filtrate were recorded at the end of every week for five weeks using Systronics pH meter.
Estimation of disulphides: Estimated after reduction of culture filtrate by sodium borohydride11.
Estimation of soluble protein concentration: Filtrate was used for determination of soluble proteins by folin phenol reagent method12with BSA as standard.
Determination of amino acids: amino acids were estimated by Ninhydrin method13.
Enzyme Assay: Keratinase activity was assayed with azokeratin as substrate. The reaction mixture contained 100 µl enzyme preparation and 800 µl 2 g1-1 azokeratin in 50 mmol 1-1 Tris buffer pH 8.0. The mixture was incubated for 15 min at 50°C and reaction is stopped by the addition of trichloroacetic acid to a final concentration of 100 g 1-1. After centrifugation at 10,000 rpm for 5 min, the absorbance of supernatant fluid was determined at 440 nm. One unit of enzyme activity was the amount of enzyme that caused a change of absorbance of 0.01 at 440 nm for 15 min at 50oC. A similar method was used to determine enzyme activity on BSA.
Analysis of soluble protein by SDS-PAGE: Filtrate was precipitated by adding solid ammonium sulphate to reach 70% saturation and was kept on an ice bath for 2 h. the mixture was centrifuged at 7,000 rpm for 30 min at 4°C. The pellet was suspended in a small volume of sample application buffer, then applied to 10% SDS-polyacrylamide gel.
Results and Discussion
The results of biochemical tests are shown in Table 2. The pH of the medium was determined at the end of every week. Fig. 2 shows the weekly change in pH. The drift in pH of medium was always towards alkalinity in all test mediums. Saturation had been reached by end of fourth week. Marked alkalization of culture medium is due to the reduction of cystine bridges (S-S) into cysteine (-SH) residues.
Table 2: Results of biochemical tests.
| S.
No. |
Wee k
in pH acids
|
Changes
disulphides
|
Release of
amino
|
Release of
soluble |
Release of
activity |
Keratinase
|
| µg/ml | µmol/ml | Proteins mg/ml | ku/ml | |||
| 1. | 1 | 7.0±0.02 | 1.04 | 85 | 0.10 | 0.5 |
| 2. | 2 | 7.35±0.02 | 1.26 | 123 | 0.23 | 1.2 |
| 3. | 3 | 7.83±0.02 | 1.85 | 186 | 0.29 | 1.9 |
| 4. | 4 | 8.14±0.02 | 2.01 | 210 | 0.31 | 2.4 |
| 5. | 5 | 8.15±0.02 | 2.03 | 215 | 0.32 |
2.5 |
 |
Figure 1: Handmade CFF paper.
|
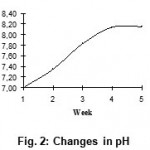 |
Figure 2: Changes in pH.
|
The release of disulphides (Fig. 3), free soluble proteins (Fig. 4), free amino acids (Fig. 5) into medium clearly indicates the keratinolytic activity of Bacillus subtilis on CFF paper. A gradual release of soluble proteins from CFF paper into the culture broth is correlated with the gradual increase in the enzyme level, which reflects again the point that alkaline protease is actually working as a keratinase. The presence of extra cellular proteolytic keratinase in the culture was estimated and found to be increasing with incubation period till fourth week (Fig. 6).
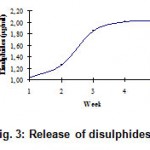 |
Figure 3: Release of disulphides.
|
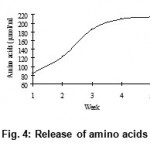 |
Figure 4: Release of amino acids.
|
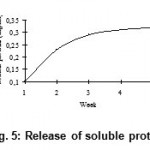 |
Figure 5: Release of soluble proteins.
|
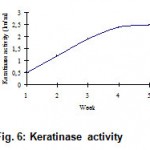 |
Figure 6: Keratinase activity.
|
SDS PAGE results (Fig. 7) show that as incubation period extended, more soluble protein bonds appeared. It was also observed that high molecular weight proteins have entered the gel and that their intensity had increased with incubation period. This is in conformity with results reported earlier by Bockle et al., that keratinase can solubilize keratin to high molecular weight derivatives and not necessarily to hydrolyze them completely14.
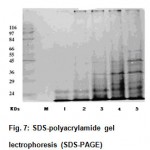 |
Figure 7: SDS-polyacrylamide gel lectrophoresis (SDS-PAGE).
|
Bio degradability of CFF paper developed by Choudary R.B. has been studied. Study of changes in pH, release of disulphides, soluble proteins and amino acids, and keratinase activity indicate that the degradation was brisk till the end of fourth week. The rate of degradation had dropped drastically during fifth week indicating that complete degradation had taken place by the end of fourth week. The study concludes that the CFF which had undergone various chemical treatments during its transformation into paper, also degrades actively under the action of B.subtilis. Active bio degradability ensures subsequent eco-friendly disposal of CFF paper products. Added to this, the abundance of protein and amino acid content present in the residue after complete degradation opens up new avenues for its usage as an additive in animal feed industry8.
References
- FAO, Chicken: population, selected indicators of food and agriculture development in Asia-Pacific region, Corporate Document Repository, Regional Office of the Asia and Pacific (2002).
- Schmidt, W.F. and Line, M.J., Physical and chemical structures of poultry feather fiber fractions in fiber process development, Nonwovens Conference TAPPI Proceedings: 135-141 (1996)
- Goddard, D.R.; Michaelis, L. J., Biol. Chem. 106: 604-614 (1934).
- Williams, C.M.; Richter, C.S.; Mackenzie, J.M.; Shih, J.C. Appl. Environ. Microbiol. 56: 1509-1515 (1990).
- Sangeetha Lal, et al., In vitro degradation of keratin by two species of Bacillus, J. Gen. Appl. Microbiol., 45: 283-287 (1999)
- Schmidt W.F., Innovative feather utilization strategies, Proceedings of the National Poultry Waste Management Symposium, Auburn, 276-282 (1998).
- Parry, D.A.D. and North, A.C.T., Hard a-keratin intermediate lament chains: substructure of the N- and C-terminal domains and the predicted structure and function of the C-terminal domains of type I and type II chains, Journal of Structural Biology., 122: 67-75 (1998).
- Nakamura Y., Wool keratin, Polymeric Materials Encyclopedia, CRC Press, USA, 2: 8769-8776 (1996)
- Noval, J.; Nickerson, W. J. Bacteriol, 77:
251-263 (1959). - Choudary R.B., et al., Keratin–Cellulose Hybrid Composites, International Symposium Research Scholars, IIT, Chennai (2007)
- Kunert, J. and Stransky, Z., Thiosulfate production from cysteine by the keratinophilic prokaryote Streptomyces fradiae, Archives of Microbiology, 150: 600-601 (1988).
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J., J. Biol. Chem., 193: 267–275, (1951).
- Sadasivam, S. and Manickam, A., Biochemical Methods, New Age Int Pub.,
40-42 - Bockle, B.; Galunsky, B.; Muller, R. Appl. Environ. Microbiol, 61: 3705–3710 (1995)
- Young, R.A.; Smith, R.E. Canadian J. Microbiol., 21: 383-386 (1975).

This work is licensed under a Creative Commons Attribution 4.0 International License.





