How to Cite | Publication History | PlumX Article Matrix
Enos M. R. Kiremire¹, Likius, S. Daniel¹, Kelly Chibale²,Henry Kambafwile2 and Philip J. Rosenthal3
1Department of Chemistry, University of Namibia, Private Bag 133 01 (Namibia) 2Department of Chemistry University of Cape Town, Rondebosch 7701 (South Africa) 3Department of Medicine, San Fransisco General Hospital, San Francisco, California 94143 (USA)
ABSTRACT: Some sulphur-based ligands and their corresponding Cu(II) and Zn(II) complexes were tested in vitro against malaria parasite. The ligands were found to be devoid of biological activity. However, the corresponding metal complexes have been found to exhibit modest biological activity against the parasite when bound to Cu(II) and Zn(II) metal ions. On the other hand, the copper(II) complex containing a ligand with thiophenecarboxylic hydrazide fragment showed quite high biological activity towards the malaria parasite. This implies that the Cu(II) and Zn(II) metal ions induced the biological activity into the ligands in question.
KEYWORDS: Antimalarial; Sulphur based ligands; biological activity
Download this article as:| Copy the following to cite this article: Kiremire E. M. R, Likius, Daniel S, Chibale K, Kambafwile H, Rosenthal P. J. Inducement of Antimalarial Biological Activity in Some S-Based Ligands by Cu(II) And Zn(II) Ions. Biosci Biotech Res Asia 2008;5(1). |
| Copy the following to cite this URL: Kiremire E. M. R, Likius, Daniel S, Chibale K, Kambafwile H, Rosenthal P. J. Inducement of Antimalarial Biological Activity in Some S-Based Ligands by Cu(II) And Zn(II) Ions. Biosci Biotech Res Asia 2008;5(1).Available from: https://www.biotech-asia.org/?p=20080 |
Introduction
In our earlier studies, we discovered that a non-active sulphur-based ligand LH when bound selected metal ions, the complexes so generated are highly active against malaria parasites1. Although the ligand was kept constant, the biological activity varied dramatically from metal to metal. The order of the biological activity varied in the order1 Cd>Zn>Mn>Co>Ni>standard>Fe. On the other hand, the biological activities of certain thiosemicarbazone ligand complexes of Cu(II), Ni(II) and Fe(III) were found to be less active against malaria parasites than the corresponding ligands2. Also relatively recently, some diacetylferrocene-derived thiosemicarbazone ligands and their Co(II), Ni(II), Zn(II), and Cu(II) complexes3 showed good biological activity against the bacterial species E. coli, B. subtillis, S. aureus, P. aeruginosa, and S. typhi as well as the fungal species T. longifusus, C. albicans, A. flavus, M. canis, F. solani, and C. glaberata. It is interesting to note that the biological activity of the ligand systems was found to be enhanced when coordinated to the metal ions3as we have found in our studies. Earlier, we discussed the paramagnetic shift influence found in the complexes Cu(LH)2Cl2, CuACl, and CuBCl relative to their corresponding ligands4-5. In our continued search for biologically active compounds against malaria parasite, monoacetylferrocene-derived thiosemicarbazones ligands shown in Figures 1-3(LH, AH, and TH) and their metal complexes[ Cu(LH)2Cl2, CuACl, and ZnT2) as well as the copper complex (CuBCl) of the 2-acetylpyridine containing thiophenecarboxylic hydrazide moiety ligand ( see Figure 4) were synthesized and tested for the biological activity. We hereby report the findings.
Experimental
The synthesis and characterization of the ligand systems, LH, AH, BH and the complexes, Cu(LH)2Cl2, CuACl, and CuBCl have already been published4-5. The synthesis and characterization of the ligand system TH and its corresponding metal complexes will be published elsewhere.
Table 1: Biological Activity Ligand qnd Metal Complex Systems.
| COMPOUND
ACTIVITY |
READING(nM)
|
SD*
|
COMMENT
ON
|
| LH | >20,000 | – | negligible |
| Cu(LH)Cl2 | 11,485 | 1,138 | modest |
| BH | not done | not done | not available |
| CuBCl | 669.9 | 6 | quite active |
| AH | > 20,000 | – | negligible |
| CuACl | 14,580 | 1,329 | modest |
| TH | > 20,000 | – | negligible |
| ZnT2 | 11,055 | 1,209 | modest |
| CQ | 90.535 | 24 | standard |
* SD = standard deviation
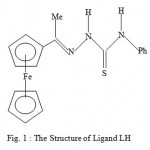 |
Figure 1 : The Structure of Ligand LH.
|
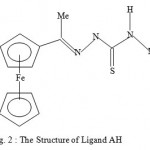 |
Figure 2 : The Structure of Ligand AH.
|
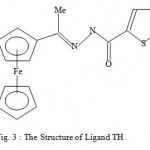 |
Figure 3 : The Structure of Ligand TH.
|
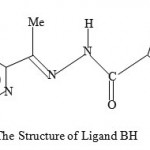 |
Figure 4 : The Structure of Ligand BH.
|
Results and Discussion
The results of the biological tests are shown in Table1. The biological tests against the malaria parasite were conducted in in vitro medium. Whereas the ligand systems LH, AH, and TH showed no biological activity, the corresponding metal complexes Cu(LH)2Cl2, CuACl and ZnT2 exhibited modest biological activity. This clearly indicates that in this case, the binding of the metal ions to the ligands either in their protonated or deprotonated forms enhances or activates the biological activity of the ligand. The copper(II) complex of the 2-acetylpyridine containing thiophenecarboxylic hydrazide moiety ligand, CuBCl was found to have very good biological activity against the malaria parasite. The mechanisms by which the metal ions activate the ligands are unclear. The search for more novel complexes with sulphur-based ligands particularly the thiosemicarbazone type must be intensified.
Acknowledgement
We wish to acknowledge the University of Namibia and Petrofund Namibia for funding the work. In addition, we wish to thank the University of Cape Town for providing facilities for the spectroscopic measurements and the University of California, San Francisco, for conducting the biological tests.
References
- M. R. Kiremire, K. Chibale, P.J. Rosenthal, L.S. Daniel, A. M. Negonga, and F. M. Munyolo, Biosciences, Biotechnology Research Asia, 4(2), 401(2007).
- Scovill, J. P., Klayman, D. L., Lambros, C., Childs, G. E., Notsch, J. D., J. Med. Chem., 27, 87(1984).
- H. Chohan, H. Pervez, K. M. Khan and C. T. Supuran, J. Enzyme Inhibition and Medicinal Chemistry, 20(1), 81(2005).
- M. R. Kiremire, L. S. Daniel, H. Mu Ashekele, and H. Kambafwile, Oriental Journal of Chemistry, 23(3),785(2007).
- M. R. Kiremire, D. S. Likius, H. Mu Ashekele, K. Chibale, and H. Kambafwile, Materials Science Research India, 4(2), 263(2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.





