How to Cite | Publication History | PlumX Article Matrix
Influence of Renal Sympathetic Denervation on Nitric Oxide-Induced Natriuresis and Diuresis
El-Sayed M. Kamel
Department of Pharmacology, Faculty of Medicine, 7th of October University, Misuratah, Libya.
ABSTRACT: Nitric oxide (NO) has been shown to influence kidney function by producing diuretic and naturutic effects . NO. also has been shown to modulate central and peripheral sympathetic activity . There for ,in order to investigate the possible role of renal sympathetic nerve on the renal effects of NO. , the effect of the No. donor, sodium nitropruoside ( SNP). Or the nitric oxide synthesis inhibitor (NOSI), IN-Nitro-l- arginin methyl ester ( L- NAME) on urine flow and renal sodium execration were studded in Sham kidney- denervated and bilaterally kidney-denervated water- loaded ethanol - anesthetized rats. In sham kidney – denervated rats, SNP. Produced significant increase urine flow and sodium execration. In contrast L- NAME caused significant reduction in urine flow and sodium execration . In kidney denervated rat ,SNP & L-NAME did not cause any significant change in the diuresis and natriuresis . This finding indicate that, the renal effects of NO. are mediated through inhibition of renal sympathetic nerves activity and thus ,has important role in regulating the neural component of renal function .
KEYWORDS: Nitric oxide; renal function; renal sympathetic nerves; Kidney-denervation
Download this article as:| Copy the following to cite this article: Kiremire E. M. R, Likius, Daniel S, Chibale K, Kambafwile H, Rosenthal P. J. Influence of Renal Sympathetic Denervation on Nitric Oxide-Induced Natriuresis and Diuresis. Biosci Biotech Res Asia 2008;5(1). |
| Copy the following to cite this URL: Kiremire E. M. R, Likius, Daniel S, Chibale K, Kambafwile H, Rosenthal P. J. Influence of Renal Sympathetic Denervation on Nitric Oxide-Induced Natriuresis and Diuresis. Biosci Biotech Res Asia 2008;5(1).Available from: https://www.biotech-asia.org/?p=20085 |
Introduction
Several studies have suggested an important role for NO in the regulation of renal function. Nitric oxide synthase, the enzyme which catalyzes the synthesis of NO, is localized in themacula densa cells, the endothelial cells of renal blood vessels, the renal pelvis and in the renal pelvic sensory nerves1,2. NO can be released in the kidney in response to various stimuli3,4 where it causes diuresis and natriuresis5,6,7. However, the mechanism (s) through which NO causes such changes in kidney function has not been fully established. The kidney receives a very dense innervation of sympathetic nerves that regulate both haemodynamic and excretory function8-10. It is well recognized that activation of renal sympathetic nerves causes vasoconstriction and reduction in renal blood flow leading to antidiuresis, and also direct enhancement of tubular reabsorption of sodium leading to antinatriuresis. There is increasing evidence indicating that NO can act as a neuromodulator of central and peripheral sympathetic activity11,12,13. Therefore, the present study aimed at investigating whether the NO-induced changes in kidney function are mediated through modulation of renal sympathetic nerves activity.
Material and Methods
Drugs and chemical used
Sodium nitropruside
Crystal, purple color .Oxford Lab. Reagent, momby. L Nitro –argenin methyl ester (L- NAME ) : powder Fluka Kemica- swizerland.
Ethyl carbamate ( Urethane )
powder , Prolabo –Paris
Ethanol : Prolabo –Paris
Animals
Adult male Albino rats ( 150-250 g. ) were used through out the study. Kidney denervation Bilateral kidney-denervation Rats (n=12) were anaesthetized with an intraperitoneal injection of urethane . ( 45 mg / kg ). A midline ventral abdominal incision approximately 2cm long was made, the renal arteries and veins isolated and any visible nerves running alongside the renal vessels were sectioned. As not all renal nerves are visible to the naked eye, 20% phenol in glycerol solution was applied with a thin brush to the renal arteries and veins to produce necrosis of all possible unsevered renal nerves, thereby ensuring a complete bilateral kidney denervation. The abdominal wall and skin were sutured and the area swabbed with 70% ethanol to prevent post-operative infection. b) Sham kidneydenervation: Rats ( n=12 ) underwent a similar surgical procedure to kidney-denervated rats except that the renal nerves were not sectioned and pure glycerol solution instead of phenol in glycerol solution was applied to the renal vessels. Diuretic experiments were performed 10 days following the surgery.
Diuretic experiments
Rats from either the kidney-denervated or the Sham kidney-denervated group were fasted for 24 h but allowed free access to water. The rats were anaesthetized with 15% ethanol (5 ml/100g) administered orally. Following naesthesia, the trachea was cannulated using a plastic cannula to allow for removal of bronchial secretions. A femoral vein was cannulated using i.v cannula for administration of drugs. The urinary bladder was exteriorized via a midline abdominal incision and cannulated using polyethylene tubing. When the cannulation procedure was completed, the rats were water-loaded by an oral dose of water equivalent to 2.5% body weight, followed 30 min later by i.v administration of a dose of 0.5% saline equivalent to 2.5% body weight. An equilibration time of 30 min was allowed and then urine samples were collected over 10 min periods and the volume measured, A stable level of hydration was maintained by rehydrating the rat with 0.7% saline administered i.v in volumes equal to the amount of urine excreted. When a constant urine flow was established for two consecutive 10 min periods, SNP ( 100 g/ kg) (5) or L-NAME ( I mg / kg) (7) were administered Iv in 0.4 ml saline, washed in with 0.2 ml saline, and urine samples collected over a 60 min period. Urine samples were stored frozen until analyzed for sodium concentration using flame photometry.
Statistical analysis
Results are presented as mean + SEM.
Data were analyzed using paired student’s t-test.
Aprobability value P< 0.05 was considered statistically significant.
Results
Effect of SNP or L-NAME in sham kidneydenervated rats
In the SNP sham kidney-denervated rats group, the control urine flow was 1.25 + 0.02 ml / 10 min, with a urinary Na+ concentration of 12.7 + 0.7 Eq/ml/100g body weight. Administration of SNP produced a diuretic effect that lasted for 50 min, with the maximal increase in urine flow (1.66 + 0.06 ml/10min ) obtained 30 min after drug administration ( Fig. 1). The diuretic effect was associated with a natriuretic effect that lasted for 30 min, with the maximal increase in urinary Na+ concentration (25.1 + 2.4 Eq / ml /100g ) obtained 20 min after drug administration (Fig. 2). In the L-NAME sham kidney-denervated rats group, the control urine flow was 1.34 + 0.03 ml / 10 min with a urinary Na+concentration of 15.1+1.3 Eq/mI/100g. Administration of L-NAME caused antidiuresis lasting for 50 min, with the maximal decrease in urine flow ( 1.07 + 0.05 ml / 10 min .) „obtained 40 min after drug administration ( Fig. 1). L-NAME also caused an antinatriuretic effect with the maximal decrease in urinary Na+ concentration ( 7.9 + 0,8 Eq/ml 100g obtained 30 min after drug administration ( Fig. 2 ). Effect of SNP or L-NAME in kidney-denervated rats In bilaterally kidney-denervated rats, the control urine flow and urinary Na+ concentration were significantly increased as compared with the sham kidney-denervated group. In the SNP and LNAME kidney-denervated rat groups, the control urine flow was 1.49 + 0.01 and 1.51 + 0.02 ml / 10 min, respectively, and the urinary Na+ concentration was 23.7 + 2.4 and 22.4+ 1.7 Eq / rut / 100g, respectively. The administration of SNP or L-NAME did not cause any significant change in either urine flow ( Fig. 3) or urinary Na+ concentration ( Fig. 4) as compared with the control values.
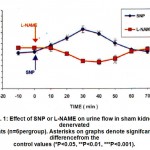 |
Figure 1: Effect of SNP or L-NAME on urine flow in sham kidney-denervated rats (n=6pergroup). Asterisks on graphs denote signifcant differencefrom the control values (*P<0.05, **P<0.01, ***P<0.001).
|
 |
Figure 2: Effect of SNP or L-NAME on urinary sodium excretion in sham kidney-denervated rats (n=6 per grotip). Asterisks on graphs dc,iiote significant dVftrc,nce from the control values ( *P<0.05, **P<0.01, ***P<0.001).
|
 |
Figure 3: Effect of’SNP or L-NAME on urine flow in kidney-denervated rats (n=6 per group ). All points were not significantly different from the control values.
|
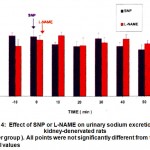 |
Figure 4: Effect of SNP or L-NAME on urinary sodium excretion in kidney-denervated rats (n=6per group ). All points were not significantly different from the control values.
|
Discussion
The present study investigates the possible interaction between renal sympathetic nerves and NO-induced changes in renal function. This was achieved by investigating the effects of the NO donor SNP or the NOS inhibitor L-NAME on urine flow and urinary Na+ excretion in water-loaded ethanolanaesthetized sham kidney-denervated and bilaterally kidney-denervated rats. The ethanolanaesthetized rat model was chosen because ethanol suppresses indogenous ADH secretion and does not directly affect renal function and therefore this model produces good urine flow which allows for continuous recording of the effects of drugs on renal excretion of water and electrolytes.(14-15). The results of the present study showed that in Sham kidney-denervated rats increasing NO concentration by administration of SNP leads to diuretic and natriuretic effects, whereas inhibition of NO synthesis by L-NAME leads to antidiuresis and antinatriuresis. These results are consistent with the findings of other studies ( 5, 6, 7 ) and confirm the role of NO in regulating the renal handling of fluid and electrolytes. In contrast, in kidney-denervated rats neither SNP nor L-NAME produced any significant change in urine flow or urinary Na+ excretion, These results indicate that the renal effects of NO are dependent on the presence of intact renal sympathetic nerves. The mechanism(s) by which NO modulates renal sympathetic effects remain to be defined, but two possibilities arise: NO might act by inhibiting neurotransmitter release from sympathetic nerve fibres or by acting within tubular epithelial cells to selectively inhibit the tubular and electrolyte transporting pathways which are regulated by catecholamine released from renal nerves. In conclusion, NO appears to be involved in the regulation of kidney function through modulation of the effect of renal sympathetic nerves activity.
Refferences
- Bandy ,R.E., Marczin, N., Chester ,AH., Yakope .N., A redox based mechanisms for nitric oxide – induced inhibition of DNA. Synthesis in humane vascular smooth muscle cells Br. J. Pharmacol. 129: 1513-1521 (2000).
- Braam B. Renal endothelial and macula densa nitric oxide synthase. Am. J. Physiol. 276: R1551- R1561 (1999).
- Matlson D, Higgins D. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension 27: 688-692 (1999).
- Valdivielso J, Crespo C, Eleno N. Renal ischaemia in the rat stimulates glomerular nitric oxide synthesis. Am. J. Physiol. 280: R771-R779 (2001).
- Peterson T, Carter A , Miller R. Nitric oxide and renal effects of volume expansion in conscious monkey, Am. J. Physiol. 291: R1033-RI038 (2003).
- Cases A, Hass J, Romero J, Burnell J. Haemodynamic and renal effects of acute and progressive nitric oxide synthesis inhibition in anaesthetized dogs. Am. J. Physiol. 280: R143-R148 (2001).
- Peterson T, Emmeluth C, Kone B, Bie P. Renal effects of nitric oxide synthase inhibition in conscious water-loaded dogs. Am. J. Physiol. 281: R584-R590 (2001).
- DiBona G. Neural control of the kidney: functionally specific renal sympathetic nerve fibres. Am. J. Physiol. 279: R1517-R1524 (2000).
- DiBona G, Kopp V Neural control of renal function. Physiol. Rev. 77: 175-179 (1997).
- Lundin S, Ricksten E. Increase in proximal tubular fluid and electrolyte reabsorption by renal nerve stimulation. Acta. Physiol. Scand.133: 455- 458 (1998).
- Zanginger J, Mohaupt N, Slhang M, Czacherk J. Inhibition of basal and reflexmediated sympathetic activity in the rostral ventrolateral medulla by nitric oxide. Am. J. Physiol. 278: R958-R962 (2000).
- Tjen S , Phan N. Nitric oxide modulates sympathoexcitatory cardiovascular reflexes. Am. J. Physiol. 281: H2010-H2017
(2001). - Xu. A. , Vita, J.F., and Keany J.Y., Ascorbic acid and glutathione modulate the biological activity of S- niro glutathione. Hypertension, 36: 291-295 (2000).
- Vazeri ,N.D., Wang, X.Q., Ovesi ,F.and Rad,B., Inductin of oxidative stress by gluta thione depletion causes sever hypertension in normal rats. Hypertension J. 36: 142-146 (2000).
- Wasam ,E., Role of renal Sympathetic nerves in nitric oxid –induced chages in renal function. JmJ., 5, 2: 143-146 (2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.





