How to Cite | Publication History | PlumX Article Matrix
Production of L-asparaginase by isolated Aspergillus species using SSF
K. B. Uppuluri1,2*, S. V. Kalidindi1, Sekhar P. V. G. V1, Harish CH1 and D. S. Rami Reddy2
1Department of Biotechnology, Bapatla engineering College, Bapatla, 52101 (India) 2Centre for Biotechnology, Au College of Engineering, Andhra University, Visakhapatnam (India)
ABSTRACT: Acute lymphocytic leukemia is a common leukemia characterized by frequent infections and anaemia. Thousands of new cases are diagnosed each year worldwide. L-asparaginase (E.C.3.5.1.1), also known as L-asparagine amino hydrolase, is a potential anti tumor enzyme that catalyses the hydrolysis of L-asparagine into L-aspartic acid and ammonia. L-asparaginase production was investigated in the filamentous fungi on sesame cake using solid state fermentation(SSF).One-factor-at-a-time approach design was applied to optimize a solid-state fermentation using the sesame cake as a main substrate, for the production of L-asparaginase by isolated Aspergillus Species. Effect of Various environmental factors like Particle size of the solid medium, Moisture content, Incubation pH, Time and temperature and number of different nutritional supplements were verified on the activity and specific activity of extracellular enzyme, L-asparaginase. Among those pH, Particle size, moisture content, glucose, Ammonium sulphate and L-asparagine were the most significant factors improving enzyme production process. The second optimization step was carried out to identify the different sources of the three factors influencing the production of enzyme namely Glucose, ammonium sulphate and L-asparagine, that bringing about maximum L-asparaginase activity. Maximal enzyme activity (191.2 IU) has been detected under the following conditions, pH 6.5, temperature 32oC, incubation period 108 hours, particle size of 0.67 cm, moisture content of 1:1 (Media: buffer) when medium was supplemented with 3%w/w Fructose, 3%w/w Ammonium sulphate, 0.1%w/w L-Asparagine, 0.01% w/w Magnesium Sulphate,01% w/w sodium chloride and inoculum size of 1.5ml (1.6 X 103Spores/ml) which is nearly three folds the activity in basal medium .
KEYWORDS: L-asparaginase production; Aspergillus niger; Sesame cake; Solid-state fermentation
Download this article as:| Copy the following to cite this article: Uppuluri K. B, Kalidindi S. V, Sekhar P.V.G.V, CH H, Reddy D. S. R. Production of L-asparaginase by isolated Aspergillus species using SSF. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Uppuluri K. B, Kalidindi S. V, Sekhar P.V.G.V, CH H, Reddy D. S. R. Production of L-asparaginase by isolated Aspergillus species using SSF. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6615 |
Introduction
L-Asparaginase the enzyme which catalyses the hydrolysis of asparagine to form aspartic acid and ammonia, is an important natural product that possess a broad spectrum of anti-tumor activity. It has been successfully applied to the treatment of several diseases such as lymphocyte sarcoma and leukemia1-3. Literally called as L-Asparagine amino hydrolase E.C 3.5.1-1, the enzyme belongs to amide group has been successfully applied to various applications. Clinical studies have demonstrated higher recovery efficiency for leukaemia patients treated with L-Asparaginase than those with IL-24. L-Asparaginase is an important chemotherapeutic agent used for the treatment of a variety of lymphoproliferative disorders and lymphomas, acute lymphoblastic leukaemia (ALL) in particular. It has been a mainstay of combination chemotherapy protocols used in treatment of pediatric ALL for almost 30 years5. Based on this, it has also been included in most contemporary, multi-agent regimens for adult ALL6,7. L-Asparaginase as a drug has demonstrated effectiveness in induction and subsequent phases of various chemotherapeutic strategies.
The pioneer observation that turned out to be important for the development of L-Asparaginase as a potential antineoplastic agent was made by Clementi 8 in 1922, revealing the presence of high activity of L-Asparaginase in the serum of guinea pig. High L-Asparaginase activity was observed only in guinea pig serum, whereas other mammals were found devoid of this enzyme. In 1953, Kidd9 described the regression of transplanted lymphomas in mice and rats by the administration of guinea pig serum. This cytotoxic activity was not present in horse or rabbit serum. Neumann and McCoy10 extended these observations in 1956. They demonstrated that the growth of cell line derived from Walker carcinosarcoma required L-asparagine. Haley et al.11 in 1961 obtained similar results, using a mouse leukaemia cell line. It was Broome in 1961 while working in Kidd’s laboratory, who compared Kidd’s finding of growth inhibition with the earliest observation by Clementi, and succeeded in concluding that the anti lymphoma activity in guinea pig sera was due to L-Asparaginase12. Further investigations of the same author confirmed its therapeutic potential13. Yellin and Wriston in 1966, succeeded in partial purification of two isoforms of L- asparaginase from the serum of guinea pig14. Interestingly, only one isoform exhibited anti lymphoma activity in vivo15. Since the extraction of this enzyme from the guinea pig serum in sufficient amounts was difficult, other sources like microbes were looked into. Mashburn and Wriston, in 1964 and Campbell and Mashburn in 1969 reported the purification of E. coli L-Asparaginase, and demonstrated its tumoricidal activity similar to that of guinea pig sera16. These findings provided a practical base for large-scale production of enzyme for pre- clinical and clinical studies17.Tumor cells, more specifically lymphatic cells, require huge amount of asparagine to keep up with their rapid malignant growth. This means they use both asparagine from the diet (blood serum) as well as what they can make themselves (which is limited) to satisfy their large L-asparagine demand. L-Asparaginase as a drug exploits this unusually high requirement tumor cells have for the amino acid asparagine. L-Asparaginase catalyses the hydrolysis of L-asparagine to L- aspartic acid and ammonia (Fig. 1).
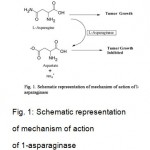 |
Figure 1: Schematic representation of mechanism of action of 1-asparaginase.
|
L-asparaginase have been produced by Escherichia coli, Serratia marcescens, Erwinia carotovora, Pseudomonas acidivoras and P. geniculata under aerobic conditions15, 18,19. The discovery of new L-asparaginase serologically different but having similar therapeutic effects is highly desired. L-asparaginase was produced throughout the world by submerged fermentation (SF)20-22. The presence of the product at low concentration and the consequent handling, reduction and disposal of large volumes of water during down stream processing in SF are known as cost intensive, highly problematic and poorly understood unit operation23. Solid-state fermentation (SSF), is a very effective technique as the yield of the product is many times higher than that in SF24 It also offers many other advantages including resistance to contamination, ease of product extraction does not require complicated methods of treating the fermented residue25. In comparison with SF, SSF offers better opportunity for the biosynthesis of low-volume-high cost products26. Screening and evaluation of nutritional and environmental requirements of microorganism is an important step for bioprocess development. Our objective was to elaborate the best conditions for production of extracellular
L- asparaginase by isolated Aspergillus niger in SSF to take the advantage of SSF specially in resisting bacterial contamination, ease of purification and the use of cheap natural materials like Sesame cake. To the best of our knowledge, this is the first report of production of L-asparaginase in SSF using sesame cake. SSF was carried out through a stepwise optimization strategy including, elucidation of medium and environmental components that affect enzyme production significantly using a One-factor-at-a-time approach, This method consists of selecting a starting point, or baseline set of levels, for each factors, then successively varying each factor over its range with the other factors held constant at the baseline level. Maximum preliminary studied are conducted using this type of strategy of experimentation.
Material and Methods
Microorganism
The fungal culture used in this study was isolated from soil sample and identified as Aspergillus niger.
Raw material
Local Sesame cake, after extracting the oil from sesame, was collected grounded as coarse powder. The ground material was collected after passing through a sieve, to attain 0.2cm particle size.
Cultivation conditions and crude enzyme extraction
After isolation of the pure culture of Aspergillus niger, a stock culture of Aspergillus niger was maintained on Potato dextrose agar slants, and were stored at 4°C. Cultivation was achieved by solid-state fermentation as reported previously27. The Fungal strain was grown for 7days at 30°C in Czapek Dox broth, from this culture conidial suspension was prepared with concentrations of 102-103 conidial spores ml-1, 1 ml of the Fungal conidial suspension was used to inoculate 10 gms of Sesame cake dispensed in 250 ml Erlenmeyer flasks. The fermentation medium has the following composition in 250 ml flask, Sesame cake, 10 g and 5 ml of 0.1 M sodium phosphate buffer (pH 7.6). Unless otherwise indicated, the flasks were autoclaved for 15 min at 121oC and inoculated with 1 ml of the previously prepared Fungal suspension. The culture flasks were then incubated at 30°C for 4 days without shaking.
The extracellular enzyme was prepared at the end of the fermentation period by the addition of 90 ml of 0.01 M sodium phosphate buffer (pH 7.0) to the medium followed by centrifugation at 8000 rpm for 20 min. The cell-free supernatant was used as crude enzyme preparation.
Protein determination
The protein content was determined according to the modified Lowry’s method28.
Enzyme assay
L-asparaginase was assayed colorimetrically29. A standard curve was prepared with ammonium sulfate. One L-asparaginase unit (IU) is defined as that amount of enzyme which liberates 1 m mole of ammonia per min under the optimal assay conditions.
Results and Discussion
Optimization of Production conditions
The effect of pH on L-asparaginase activity was determined. The pH ranges from 5.5 to 8 was employed. pH 6.5was found to be optimum for the L-asparaginase production (Fig.2). The influence of Fermentation time was shown in Fig.3.
The maximum Activity and specific activity of L-Asparaginase was observed as 75.25 IU at 108 hours. To determine the incubation temperature, flasks were incubated at different temperatures ranging from 24 to 44°C. At 32°c incubation temperature was found to be optimum with 75.5 IU of enzyme activity (Fig.4).
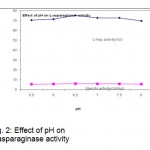 |
Figure 2: Effect of pH on L-asparaginase activity.
|
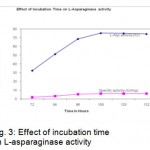 |
Figure 3: Effect of incubation time on L-asparaginase activity.
|
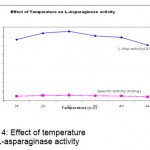 |
Figure 4: Effect of temperature on L-asparaginase activity.
|
Effect of different supplements was studied by the addition of carbon nitrogen, growth promoter and different mineral salts were added and checked for the L-Asparaginase enzyme activity. Some supplements like glucose, ammonium sulphate, L-Asparagine, Magnesium sulphate and sodium chloride have shown the positive effect, some are negative effect and some have shown the neutral effect. Addition of those positively influenced supplements improves the enzymatic activity from 75.5 IU to 121.7 IU. Further each ingredient in the production medium was optimized. The L-Asparaginase activities were considerably increased with the change of each ingredient concentration. Table.1 clearly indicated that the L-Asparaginase activity and specific activity can be increased from 121.7 IU and 13.003 IU/mg when the basal medium added with the supplements, to 191.2 IU and 19.877 IU/mg with optimization of all supplements with the medium.
Analysis of variance showed that there was significant improvement in the L-Asparaginase activity when the sesame cake was supplemented with the different carbon, nitrogen and growth promoters. Glucose, fructose, and sucrose were suitable carbon sources for the L-Asparaginase activity with the 172.5, 191.2 and 165.6 IU respectively (Table 2). Five different Nitrogen sources (Ammonium Sulphate, Ammonium Phosphate, Ammonium Chloride, Urea and Yeast extract) and five different Growth promoters
(L-Asparagine, Tryptophan, Phenyl alanine, Thiamine and Folic acid) were used to supplement the Sesame cake for the L-Asparaginase activity. Ammonium sulphate (191.2 IU), Yeast extract (173.6 IU), L-Asparagine (191.2 IU) and Tryptophan (176.4 IU) have significantly influenced the
L-Asparaginase activity with Sesame cake as a sole substrate. Maximal enzyme activity (191.2 IU) has been detected when medium was supplemented with 3%w/w Fructose, 3%w/w Ammonium sulphate, 0.1%w/w L-Asparagine, 0.01% w/w Magnesium Sulphate,01% w/w sodium chloride and inoculum size of 1.5ml (1.6 X 103Spores/ml) which is nearly three folds the activity in basal medium (Table 1)
Table 1: Optimization of environmental and additional nutrient factors in Sesame cake for L-Asparaginase activity with isolated Aspergillus Species.
|
S. Enviomental/Nutrient Concentration L-Asp. SA Concentration L-Asp. SA |
| No. Ingredient added used with Activity (IU/mg) optimized Activity (IU/mg) |
| sesame cake y(IU) Y(IU) |
| 1 With out any supplements – 75.21 9.27 |
| 2 Particle size 0.2 cm 121.7 11.753 0.67cm 132.4 13.003 |
| 3 Moisture content 2:1(Cake: 121.7 11.753 1:1(Cake: 141.2 14.032 |
| Buffer) Buffer) |
| 4 Fructose 2%W/W 121.7 11.753 3%W/W 155.7 15.727 |
| 5 Ammonium sulphate 2%W/W 121.7 11.753 3%W/W 162.1 16.475 |
| 6 L-Asparagine 0.1%W/W 121.7 11.753 0.2%W/W 181.4 18.731 |
| 7 Magnesium Sulphate 0.01%W/W 121.7 11.753 0.01%W/W 189.4 19.66 |
| 8 Sodium chloride 0.01%W/W 121.7 11.753 0.01%W/W 189.4 20.364 |
| 9 Amt. of inoculum 1ml 121.7 11.753 1.5ml 191.2 19.877 |
| (1.6X102 Spores/ml) |
| SA: Specific activity |
Table 2: Effect of supplementary Additions in Sesame.
| cake as carbon sources on L-Asparaginase activity | |||
| S.No Carbon source(3%w/w) L-Asparaginase activity(IU) Specific activity (IU/mg) | |||
| 1. | Glucose | 172.5 | 17.691 |
| 2 | Fructose | 191.2 | 19.877 |
| 3 | Maltose | 140.5 | 13.950 |
| 4. | Lactose | 148.6 | 14.897 |
| 5. | Sucrose | 165.6 | 16.884 |
Conclusion
In screening the factors affecting production of certain secondary metabolites it is very important to test as many factors as possible and to identify the significance of each of these. One-factor-at-a-time approach design offers a good procedure for computing the significance of individual factors and maintains convincing information on each component. Although, other interactions are not included in this design, it is the first priority in the screening program to examine the effect of individual factors, both environmental factors like Particle size of the solid medium, Moisture content, Incubation pH, Time and temperature and nutritional factors on the accumulation of Extracellular enzyme, L-asparaginase. Of these, only the most effective factors with positive significance would be selected for further optimization, in which different alternative sources were screened for the better accumulation of enzyme.
Our preliminary studies had shown that, a better activity of enzyme was achieved at a pH of 6.5 and temperature of 32°C after 108 hours of incubation with a particle size of 0.67 cm by adding moisture content of 1:1 (Media: buffer). Various nutritional factors like Carbon, Nitrogen, growth factors, and mineral sources had been screened among which carbon, nitrogen and growth factors shown positive influence on the enzyme activity where as other factors like mineral sources had no influence. The present work was significant as it paves a new era for the production of L-Asparaginase using a cheap medium like Sesame cake from fungal strains. It is useful to advise microbial industry sponsors to adopt such process to maintain high efficiency and economic bioprocesses There is lot of scope for future work in many aspects like examination of the interaction between a large number of variables by applying the statistical methods, which converts the bioprocess factor correlations into a mathematical model that predicts where the optimum is likely to be located.
Acknowledgements
We are thankful to Bapatla Education Society, Bapatla for providing the necessary lab facilities to carry out the research work.
References
- Yamada K, Hirano M, Kakizawa H, Morita A, Uetani T. Antileukemic action of L-Asparaginase. Saishin Igaku, 25: 1064-74 (1970).
- Adamson RH, Fabro S. Antitumor activity and other biologic properties of L-Asparaginase (NSC-109229)-A review. Cancer Chemother Rep, 52: 617-22 (1968).
- Haskell CM, Canellos GP, Cooney DA, Hardesty CT. Pharmacologic studies in man with crystallized L-Asparaginase (NSC-109229). Cancer Chemother Rep, 56:
611-4 (1972). - Carbone PP, Haskell CM, Leventhal BG, Block JB, Selawry OS. Clinical experience with L-Asparaginase. Recent Results Cancer Res, 33: 236-43 (1970).
- Ortega JA, Nesbit Jr ME, Donaldson MH, et al. L-Asparaginase, vincristine and prednisone for induction of first remission in acute lymphoblastic leukaemia. Cancer Res, 37: 535–40 (1977).
- Gokbuget N, Hoelzer D. Recent approaches in acute lymphoblastic leukaemia in adults. Rev Clin Exp Hematol, 6(2):114–41 [discussion 200–202] (2002).
- Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukaemia: cancer and leukaemia group B study 8811. Blood, 85(8):2025–37 (1995).
- Clementi A. La desemidation enzymatique de l-asparagine chez les differentes especes animales et la signification physiologique de sa presence dans l organisma. Arch Int Physiol, 19: 369–76 (1922).
- Kidd J. Regression of transplanted lymphoma induced in vivo by means of normal
guinea pig serum I. J Exp Med, 98: 565–82 (1953). - Haley EE, Fischer GA, Welch AC. The requirement for lasparagine of mouse leukaemia cells L5178Y in culture. Cancer Res., 21: 532–41 (1961).
- Broome JD. Evidence that the L-Asparaginase activity in guinea pig serum is responsible for its antilymphoma effects. Nature. 191: 1114–5 (1961).
- Broome JD. Evidence that the L-Asparaginase of guinea pig serum is responsible for its antilymphoma effects I.
J Exp Med, 118: 99–120 (1963). - Yellin TO, Wriston JC. Purification and properties of guinea pig serum L-Asparaginase. Biochemistry, 5: 1605–12 (1966).
- Yellin TY, Wriston JC. Antagonism of purified asparaginase from guinea pig serum towards lymphoma. Science, 151: 998–1004 (1966).
- Mashburn L, Wriston JC. Tumor inhibitory effects of L-Asparaginase from Escherchia coli. Arch Biochem Biophys, 105: 450–2 (1964).
- Campbell H, Mashburn L, Boyse E, Old JL. Two L-Asparaginases from E. coli B, their separation, purification and antitumor activity. Biochem Genet. 6: 721–30 (1967).
- Oettgen RF, Old JL, Boyse EA, et al. Inhibition of leukaemias in man by L-Asparaginase. Cancer Res, 27: 2619–31 (1967).
- Distasio JA, Niederman RA, Kafkewitz D, Goodman D. Purification and characterization of L-asparaginase with anti- lymphoma activity from Vibrio succinogenes. J Biol Chem, 251: 6929/33 (1976).
- Dey SK, Raha SK, Roy SK, Chakrabarty SL. Comparison of L- asparaginase activity in different species of Vibrio . Indian J Med Res 88: 398/403 (1988).
- Albanese E, Kafkewitz K. Effect of medium composition on the growth and asparaginase production of vibrio succinogenes. Appl Environ Microbiol., 36: 25/30 (1978).
- Mostafa SA, Salama MS. L-asparaginase -producing Strepto- myces from the soil of Kuwait. Zentralbl Bakteriol Naturwiss, 134: 325 /34 (1979).
- Radcliffe CW, Kafkewitz D, Abuchowski A. Asparaginase production by human clinical isolates of Vibrio succinogenes.
Appl Environ Microbiol, 38: 761/2 (1979). - Datar R. Economics of primary separation steps in relation to fermentation and genetic engineering. Process Biochem, 21: 19/26 (1986).
- Arima K. Microbial enzyme production. In: Starr MP, editor. Global impacts of applied Microbiology, 279/94 (1964).
- Lonsan BK, Ghildyal NP, Budiatman S, Ramakrishna SV.Engineering aspects of solid-state fermentation. Enz Microb Technol, 7: 258/65 (1985).
- Balakrishnan K, Pandey A. Production of biologically active secondary metabolites in solid state fermentation. J Sci Ind Res, 55: 365/72 (1996).
- Olama ZA, El-Sabaeny AH. Lipase production from Aspergillus niger under various growth conditions by solid state fermenta- tion. Microbiologia SEM 9: 134/41 (1993).
- Ohnishi ST, Barr JK. A simplified method of quantitating protein using the biuret and phenol reagent. J Anal Biochem, 86:
193/200 (1978). - Sinha A, Manna S, Roy SK, Chakrabarty SL. Induction of L- asparaginase synthesis in Vibrio proteus. Indian J Med Res., 93: 289/92 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





