How to Cite | Publication History | PlumX Article Matrix
S. Murugan1*, P. Uma Devi2, P. Mahesh1, S. Suja2 and K.R. Mani3
1Post Graduate and Research Department of Microbiology,Dr. N. G.P. Arts and Science College, Coimbatore - 641 035 India.
2Post Graduate and Research Department of Biochemistry, Dr. N. G.P. Arts and Science College, Coimbatore - 641 035 India.
3Central Research Institute, Kasauli - 173 204 India.
Corresponding Author E-mail: micro_murugs@inbox.com
ABSTRACT: Presence of high tannin contents in pomegranate and grapes are responsible for haze and sediment formation as well as colour, bitterness and astringency of the juice upon storage. Due to the inability of conventional fruit juice debittering process for removing the bitterness effectively, enzymatic debittering should be preferred. This present work is aimed at the debittering of pomegranate and grape juices using tannase, which has potential applications in degradation of tannins. The juice was treated with tannase 0.432Ug-1 at 37°C for two hour and then the enzyme was inactivated at 50°C for 30 minutes. Concentration of enzyme and the time of treatment also plays a crucial role in the debitterness. Tannase treatment resulted in 55% degradation of while a combination of tannase and gelatin (1:1) resulted in 60% of tannin degradation. Ascorbic acid, pH, total sugar, and protein content of both the treated and non-treated fruit juices were studied.
KEYWORDS: Pomegranate; grape; tannase; debittering; tannin degradation; ascorbic acid content
| Copy the following to cite this article: Murugan S, Devi P. U, Mahesh P, Suja S, Mani K. R. Production of tannase by citrobacter freundii under solid state fermentation and its application in fruit juices debittering. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Murugan S, Devi P. U, Mahesh P, Suja S, Mani K. R. Production of tannase by citrobacter freundii under solid state fermentation and its application in fruit juices debittering. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6753 |
Introduction
Tannase catalyses the hydrolysis of gallic acid and hydrolysable tannins. Plants and microorganisms produce this enzyme, which is industrially used as a catalyst in the manufacture of gallic acid. Industrial bioconversion of tannic acid is generally accomplished by enzyme tannase for the production of gallic acid (3, 4, 5 – trihydroxy benzonic acid). Gallic acid is mostly utilized in the pharmaceutical industry for manufacture of trimethoxy benzaldehyde, which is used in the production of a broad – spectrum antibiotic, trimethoprim (Bajpai and Patil 1996). It is also potentially used in beverage and fruit juice processing. The pomegranate is one of the oldest known edible fruits, affectionately known as the “jewel of winter” has recently been acclaimed for its health benefits, in particular, for its disease-fighting antioxidant potential. Grapefruit is an excellent source of vitamin C, a vitamin that helps to support the immune system. Vitamin C-rich foods like grapefruit may help reduce cold symptoms. Vitamin C also prevents the free radical damage that triggers the inflammatory cascade, and is therefore also associated with reduced severity of inflammatory conditions, such as asthma, osteoarthritis, and rheumatoid arthritis
There is an increased concern in the fruit juice industry about the loss of market value of pomegranate and grape juices due to its haziness and bitterness because of high tannin content (Vardin and Fenercioglu 2003). More over the negative effect of tannins in animal nutrition is due to their capacity to bind macromolecules and therefore rendering them indigestible (Belmers et al. 2004). There are several potential industrial applications for tannase; however, due to the high production costs and limited knowledge of its catalytic action, there are currently only a few applications (Lekha and Lonsane 1997; Batra and Saxena 2005). Adsorption techniques have been reported to achieve the role of debittering in several fruit juices (Chien et al. 2001). Because of loss of acidity, sweetness, flavour and turbidity as well as efficiency in adsorption debittering, enzymatic hydrolysis has showed superior potential in industrial application (Puri et al. 1996). In manufacture of beer, tannase is used extensively to reduce the tannin content. Based on the potential use of tannase to reduce tannin levels in fruit juices, the aim of this work was to reduce the bitterness of pomegranate and grape juices without a significant loss in the quality. The current study aims to process fruit at the lowest possible cost while maintaining the quality and stability of the finished product.
Materials and Methods
Chemicals
All the chemicals used for the following experiments were of analytical grade.
Microorganism
Citrobacter freundii which is used for the production of tannase enzyme was obtained from IMTECH (Strain No: NCTC 9750),Chandigarh, India. The culture was maintained on nutrient agar slant at 40C. It was sub – cultured at four week intervals.
Substrates
Caesalpinia dygina fruit powder, Terminalia chebula cover powder and Madhuca indica bark powder were collected from local market of Coimbatore, Tamilnadu, India and dried under shadow for 24 hours. The dried materials were grounded to fine powder and stored in polythene bags at room temperature (30±2°C).
Sample collection
Fresh pomegranate (Punica granatum) and grape fruits (Citrus paradise) were procured from the local market of Coimbatore, Tamilnadu, India. The fruits chosen were at the mature stage with firm texture, uniform color and no sign of spoilage.
Inoculum preparation and inoculation
100ml sample of Nutrient broth solution with 2% nutrient broth (Hi-Media, India) was sterilized at 1210C for 15min, cooled and was inoculated under aseptic condition with Citrobacter freundii (Strain No: NCTC 9750) from Nutrient agar slant. The broth culture was incubated for 24 hours on rotary shaker (130 rpm) at 370 C. A 10ml of aliquots for 100ml production medium is used as its inoculum (seed culture).
Enzyme production in Solid State Fermentation (SSF)
Erlenmeyer flask [250ml] containing 10g of Caesalpinia dygina fruit powder, 10g of Terminalia chebula cover powder, 10g of Madhuca indica bark powder(1:1:1:) was added to the mineral salt medium [gl-1, Na2HPO4.2H2O-1.1; NaH2PO4.2H2O – 0.06 ; KCl-0.3; MgSO4.7H2O-0.01; pH 7.0] were autoclaved at 1210C for 20 min, cooled to about 300C and inoculated with a 10%(v/w) inoculum. The content of the flasks were mixed thoroughly to ensure uniform distribution of the inoculum and incubated in a shaker at [130 rpm] 370C for 48 hours. Samples were drawn at 12 hours interval and the content was extracted with 0.01 M phosphate buffer (pH 7.0). The extract was centrifuged at 8000 rpm for 20 min at 40C. The clear supernatant obtained was used as enzyme source.
Acetone precipitation of tannase
In order to use the partially purified enzyme in juice debittering, the enzyme was mixed with acetone at 40 C in 1:2 volumes and after 4 hours of incubation, the enzyme and acetone mixture was centrifuged at 10,000 rpm for 10 minutes. The precipitate was dissolved in minimum amount of buffer to get partially purified tannase.
Tannase assay
Tannase activity was determined colorimetrically, according to the method of modified Mondal et al. (2001). One unit (U) of enzyme activity is defined as the amount of enzyme required to hydrolyze 1µmol of ester in minute to get the partially purified tannase.
Juice preparation
Fruits were washed with water to remove any adhering substances. The rind, rag and membrane of the fruit sacs were removed mechanically. Juices were extracted from the arils by homogenizing in a blender followed by filtration through cheesecloth. The extracted juice was stored at 40 C until used.
Preparation of gelatin
Gelatin was pre-swelled by dissolving 1 gm of gelatin in 1 liter of distilled water and heated at 600 C for 2 hours. 1:1 volume of gelatin and tannase was mixed just before treatment.
Treatment of juice with tannase
To 10 ml of fruit juice taken in a test tube, 1.5 ml of tannase (0.432Ug-1) was added. The control test tube received only 1.5ml of water instead of tannase. Test tube was then incubated at 370 C with gentle shaking up to 120 minutes. The test tube was then placed in a water bath at 500 C for 10 minutes to deactivate the enzyme. 1ml of aliquot of fruit juices was taken from each test tube at different time intervals and their tannin content was measured.
Tannin content estimation
The tannin content in the fruit juices of pomegranate and grape was measured following the protein precipitation method by tannins (Gao et al. 1978).
Protein estimation
Protein was estimated following Lowry’s method (Lowry et al. 1951).
Carbohydrate estimation
The total carbohydrate content was determined by phenol-sulphuric acid method (Dubois et al. 1956).
Total phenolics estimation
Total phenolic estimation was carried out with Folin-Ciocalteau reagent
(Chaterjee et al. 2004).
Determination of pH
pH of the juice was determined using pH meter.
Ascorbic acid content
To 25 ml of both treated and non treated fruit juice sample, 1ml of the starch solution was added. Then it was titrated against iodine solution. The ascorbic acid content was calculated from a standard curve of ascorbic acid.
Determination of elements by Atomic Absorption Spectrophotometer (AAS)
For the determination of elements by AAS, 5ml of fruit juice was digested with 25ml of nitric acid and perchloric acid (9:4) for 1 hour. The digested fruit juice was filtered through a Whatman no 42 filter paper and the final volume was made to 100ml. Element content of the digested juice was determined by AAS.
Sensory evaluation
The organoleptic evaluation of different juices for assessing the quality of juices after treatment by a panel of eight judges. Juices were supplied to them in dark bottles to avoid any visual bias.
Results and Discussion
To achieve optimum tannase production various parameters viz. pH, temperature and incubation period for pure culture of Citrobacter freundii were optimized. The optimum tannase production parameters for pure culture of Citrobacter freundii were pH 7.0, temperature 40˚C and incubation period 48 hours. When different concentration of tannase (0 to 2ml ) on tannin degradation in both pomegranate and grape juices studied , it was found that 1.5ml ( 0.432 Ug¯¹) of tannase showed maximum degradation of tannin in both juices after 120 minutes of incubation (Figure 1). 1.5ml of tannase (0.432Ugˉ1) was used to clarify 10 ml of fruit juices. A further increase in tannase concentration did not improve the extent of clarification for both the juices.
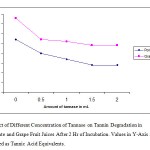 |
Figure 1: Effect of Different Concentration of Tannase on Tannin Degradation In Pomegranate and Grape Fruit Juices After 2 Hr of Incubation. Values in Y-Axis Are Represented As Tannic Acid Equivalents.
|
When the fruit juices (10ml) of pomegranate and grapes tested with 1.5ml (0.432 Ug¯¹) of tannase enzyme at different incubation period (0-120 minutes), it was found that the maximum degradation of tannin achieved at 100 minutes (Figure 2). A 55% decrease in tannin content was observed after 2 hours of incubation with the enzyme at 40˚C.This indicated that debittering efficiency depends on the concentration of enzyme used and the incubation period. The effect of gelatin (1g/L) and tannase (0.432Ug-1) in 1:1 ratio was also tested for tannin degradation at various time intervals up to 2 hours. The gelatin and tannase combination showed a sharp decline in tannin level in fruit juices up to 60% after 100 minutes of incubation (Figure 3). Such decrease in tannin level by gelatin and enzyme treatment may be attributed to the ability of gelatin to precipitate tannins.
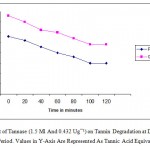 |
Figure 2: Effect Of Tannase (1.5 Ml And 0.432 Ug¯¹) On Tannin Degradation At Different Incubation Period. Values In Y-Axis Are Represented As Tannic Acid Equivalents.
|
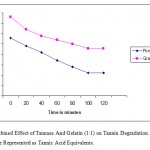 |
Figure 3: Combined Effect of Tannase and Gelatin (1:1) on Tannin Degradation. Values in Y-Axis Are Represented As Tannic Acid Equivalents.
|
In order to determine the change in quality due to the treatment with debittering acids, the juices were subjected to analysis of ascorbic acid content, carbohydrate, protein, pH and titrable acidity (Table 1). Non- treated and treated samples did not show any significant variation in these aspects. However, there was an increase in total sugar content from 3.2mg in untreated pomegranate juice to 5.7mg in treated juice, similarly 4.7 mg in untreated grape juice to 5.9 mg in treated juice. Both the treated and untreated juices were analyzed for the presence of essential elements. There were no significant differences in the content of iron, aluminum, copper, and zinc (Table 2). Sensory evaluation of the juices before and after treatment was performed on the basis of quality parameter like flavour, appearance, color and acceptability. It was evaluated that the acceptance of the juice has increased after treatment. Citrobacter freundii was used as inoculum for the bioconversion of the tannin rich substrate to tannase. A 55% decreases in tannin content was achieved after 2 hours of incubation as tannase catalyses the hydrolysis reaction of the ester bond present in the hydrolysable tannins and gallic acid esters. Cherry juice treatment with gelatin and silica solution followed by high- speed centrifugation resulted in improve clarity of fruit juice by 99% (Meyer et al. 2001). However, no report on the attempt of the tannin reduction with pomegranate and grape juices by bacteria. In the present study, an attempt has been made for the degradation of tannin, which is responsible for the bitterness of the pomegranate and grape juice by enzyme and gelatin combination. A decrease in tannin content by 49% with the above treatment could be attributed to the ability of gelatin to precipitate tannin (Basil et al. 1995). The combined effect of hydrolysis and precipitation of tannin by tannase and gelatin respectively resulted in higher tannin degradation.
Table 1: Detection of Important Elements in Treated and Non-Treated Fruit Juices.
In the present study, it was also noticed that there was a considerable increase in sugar content in the treated fruit juices. The authors hypothesis that because of the action of enzyme, tannin acyl hydrolysis otherwise known as carbohydratase, acts on the complex sugar moieties present in the fruit juices and release free sugars in the juices. The hydrolysis with tannase results into gallic acid and glucose, which increased the total sugar content of the treated fruit juices by reducing the tannin content (Lekha and Lonsane 1997). An increase of 20% in reducing sugar content using a combination of pectic enzyme and cellulases was reported for the clarification of apple juice (Floribeth and Lastreto 2004).Analysis of vitamin C content as another in citrus juices after filter-aids treatment has been reported by a number of researchers, from no measurable loss up to 33% loss, depending on the tubes of filter-aids and processing conditions (Matthews et al. 1990). The present study suggests that partially purified tannase in acetone could be used as a clarifying aid of fruit juices. However, a combination of gelatin and tannase was found to be superior for its debittering activity as compared to the enzyme alone and treatment has no negative impact on the biochemical and quality attributes of the fruit juices.
Acknowledgement
The authors are grateful to Dr. Thavamani D. Palanisamy, Secretary of Dr.N.G.P. Arts and Science College and other members of KMCRET, Coimbatore for providing laboratory facilities and encouragements.
References
- Bajpai, B. and Patil, S. 1996. Tannin acyl hydrolase activity(EC 3.1.1.20) of Aspregillus, Penicillium, Fusarium and Trichoderma. World J.Microbiol. Biotechnol. 12, 217-220.
- Basil, I.M., Maia, G.A. and Figueiredo, R.W. 1995. Physical- Chemical changes during extraction and clarification of guava juice. Food. Chem. 54, 383-386.
- Batra, A. and Saxena, R.K. 2005. Potential tannase production from the genera Aspergillus and Penicillium. Process Biochem. 40, 1553-1557.
- Belmers, R., Carlos, J., Esquivel, C., Harrera, R. R., Coronel, A .R. and Aguilar, C.N.2004. Microbial production of tannase: An enzyme with potential use of food industry. J. Food Sci. Technol. 37, 857-864.
- Chaterjee, S., Chaterjee, B.P.and Guha, A. K. 2004. Clarification of fruit juice with chitosan, Process Biochem. 39, 2229-2232.
- Chien, J.P., Sheu, F. and Shyu, T. 2001. Monitoring of enzymatic debittering of grape fruit juice by high performance liquid chromatography. J.Food Drug Anal.9, 115-120.
- Dubois, M., Gilles, K. A. and Hamilton, J.K. 1956. Colorimetric method of determination of sugar and related compounds. Anal. Chem. 26,350.
- Floribeth, V. and Lastreto, C.A. 2004. A study of the production of clarified banana juice using pectinolytic enzymes. J.Food Technol. 16, 115-125.
- Gao, X. Q., Ohlander, M., Jeppsson, N., Bjork, L. and Trajkovskl, V.2000. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophaerhamnoides L.) during maturation. J. Agric. Food Chem. 48, 1485-1490.
- Mondal,K.C., D.Banerjee, M.Jana and B.R.Patil, 2001.Colorimetric assay method for the determination of the tannin acyl hydrolase (E.C.3.1.1.20) activity. Anal.Biochem.,295:168-171.
- Lekha, P.K. and Lonsane, B. K. 1997. Production of application of tannin Acyl Hydrolase: state of the art. Adv. Appl. Microbiol. 44, 215-260.
- Lowry, O. H., Rosenbrough, N. J., Farr, A.L. and Randall, R.J. 1951.Protein measurement with folin phenol reagent. J. Biol .Chem. 193, 269-275.
- Matthews, R.F., Rouseff, R. L., Manlan, M. and Norman, S.L. 1990. Removal of limonene and narigin from citrus juice by styrene-divinylbenzene resins. Food Technol. 44, 130-132.
- Meyer, A. S., Koser, C. and Nissen, J. A. 2001. Efficiencuy of enzymatic and other alternative clarification and fining treatments on turbidity and haze in cherry juice. J. Agric. Food Chem. 49, 3644-3650.
- Puri,M., Marwaha,S.S., Kothari,R.M. and Kennedy,J.F. 1996.Biochemical basis of bitterness in citrus fruit juices and biotech approaches for debittering. Crit. Rev.Biotechnol. 16, 1465-155.
- Vardin, H. and Fenercioglu, H. 2003. Study on the development of pomegranate juice processing technology: clarification of pomegranate juice. Nahrung. 47, 300-303.

This work is licensed under a Creative Commons Attribution 4.0 International License.





