How to Cite | Publication History | PlumX Article Matrix
M. Vijey Aanandhi1*, Shiny George1 and V. Vaidhayalingam2
1Department of Pharmaceutical Chemistry, Vel’s College of Pharmacy, Chennai - 600 117 India.
2Department of Pharmaceutical Chemistry, Madras Medical College, Chennai - 600 003 India.
ABSTRACT: Some novel Mannich base isatin derivatives were synthesized by reacting 1-(5-methoxy-2-oxoindolin-3-ylidene)-4-(substituted pyridin-2-yl)thiosemicarbazide with formaldehyde and several secondary amines. Their chemical structure was elucidated by means of spectral (FT-IR, 1H NMR and mass) analysis. Investigation of analgesic activity of synthesized compounds was done by acetic acid induced writhing reflex method using diclofenac sodium as standard drug. The synthesized compounds showed significant analgesic activity.
KEYWORDS: Isatin; Schiff base; Mannich base; analgesic activity
Download this article as:| Copy the following to cite this article: Aanandhi M. V, George S, Vaidhayalingam V. Synthesis and Analgesic Activity of 1-(1-((Substituted) Methyl)-5- Methoxy-2-Oxoindolin-3-Ylidene)-4- (Substituted Pyridin-2-Yl)Thiosemicarbazide. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Aanandhi M. V, George S, Vaidhayalingam V. Synthesis and Analgesic Activity of 1-(1-((Substituted) Methyl)-5- Methoxy-2-Oxoindolin-3-Ylidene)-4- (Substituted Pyridin-2-Yl)Thiosemicarbazide. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6843 |
Introduction
Isatin (1H-indole-2, 3-dione) was first obtained by Erdman and Laurent in 1841 as a product from the oxidation of indigo by nitric and chromic acids. Isatin is an endogenous compound identified in human that possesses wide range of biological activities. Isatin has anxiogenic¹, anticonvulsant², anticancer² activity and act as a potent antagonist on atrial natriuretic peptide receptors in vitro³. Isatin derivatives of Mannich bases have antibacterial4, antifungal5, antiviral6, anti HIV7-9 and antiallergic10 activity. In addition pyridines are associated with diverse biological activities11,12. The N-Mannich bases of isatin derivatives were synthesized by condensing the Schiff base of isatin derivative with formaldehyde and various secondary amines (Scheme 1). The structures, yields and melting points of the synthesized compounds are given in Table 1. All the compounds gave satisfactory elemental analysis. IR, 1H NMR and mass spectra were consistent with the assigned structures.
All the synthesized compounds were screened for analgesic activity.
Material and Methods
All chemicals used in the study were supplied by E. Merck and SD fine. Melting points were taken in a Thomas Hoover capillary melting point apparatus and are uncorrected. The purity of the synthesized compounds was routinely checked by TLC on silica gel G. IR spectra on a Shimadzu FT 8300 infrared spectrophotometer (umax cm-1), 1H NMR spectra were recorded on JEOL GSX 400 spectrometer using TMS as an internal standard (chemical shifts in ä, ppm) and mass spectra on a JEOL MSMATE spectrometer.
Experimental
Synthesis of 5-methoxy isatin (1)
To 65ml of concentrated sulphuric acid at 50°C was added 15 g of dry isonitrosoacetanilide derivative. The solution was heated to 80°C and was kept at this temperature for about 10 minutes. Then it was cooled to room temperature and poured upon cracked ice. After 90 minutes, the 5-methoxy isatin was filtered, washed several times with cold water to remove sulphuric acid and then dried in air.
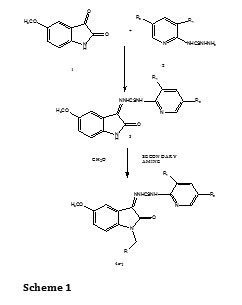
Synthesis of 4-(substituted pyridin-2-yl) thiosemicarbazide (2)
To a solution of 2- amino 3- methyl pyridine (0.01mol) in DMF (10ml) was added sodium hydroxide (0.01mol) and carbon disulphide (0.75ml). The mixture was stirred at 15-20°C for 1 hour, to the stirred mixture was added hydrazine hydrate (0.01mol) and stirring continued at 60°C for 1 hour. On adding water a pale yellow solid separated out which is recrystallized from DMF and ethanol. Similarly other derivative was synthesized.
Synsthesis of 1-(5-methoxy-2-oxoindolin-3-ylidene)-4-(substitutedpyridin-2-yl) thiosemicarbazide (3)
Equimolar quantities of 5-methoxy isatin and 4(substituted pyridin-2-yl) thiosemicarbazide were dissolved in warm ethanol (50ml) containing 1ml of glacial acetic acid. The reaction mixture was refluxed for 10 hours and set aside. The resultant solid was washed with dilute ethanol, dried and recrystallized from ethanol and chloroform mixture.
Synthesis of compound (4a-4j): A slurry consisting of 1-(5-methoxy-2-oxoindolin-3-ylidene)-4-(substituted pyridin-2-yl) thiosemicarbazide (0.002 mol), THF (3ml) and 37% formalin (1ml) was made. To this secondary amine (0.003mol) was added drop wise with cooling and shaking. The reaction mixture was allowed to stand at room temperature for 1hour with occasional shaking after, which it was warmed on a steam bath for 15minutes. At the end of the period the contents were cooled and the product obtained was recrystallized from chloroform and petroleum ether.
1-(1-((dimethylamino)methyl)-5-methoxy-2-oxoindolin-3-ylidene)-4-(3-methylpyridin-2-yl) thiosemicarbazide (4a) the sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3365, 2850, 1690, 1645, 1280, 1105 cm-1 ;1H NMR (DMSO d6): d 8.09 (d, H), 7.6 (d, H), 7.38 (d, H), 7.1 (d, H), 7.0 (s, H), 6.8 (m, H), 6.51 (m, H), 4.03 (s, 2H), 4.0 (s, H), 3.73 (s, 3H), 2.32 (s, 3H), 2.27 (m, 6H); MS (relative intensity): m/z value 398.15 (23); Calcd. for C19H22N6O2S % C 57.27 H 5.56 N21.09; found C 57.25 H 5.54 N 21.06
1-(1-((diethylamino) methyl)-5-methoxy-2-oxoindolin-3-ylidene)-4-(3-methyl pyridin-2-yl) thiosemicarbazide (4b)
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3370, 2854, 1700, 1640, 1284, 1110 cm-1 ;1H NMR (DMSO d6): d 8.09 (m, H), 7.6 (d, H), 7.38 (d, H), 7.1 (d, H), 7.0 (s, H), 6.8 (t, H), 6.51 (d, H), 4.03 (s, 2H), 4.0 (s, H), 3.73 (s, 3H), 2.40 (m, 4H), 2.32 (s, 3H), 1.00 (m, 6H); MS (relative intensity): m/z value 428.18(25) ; Calcd. for C21H26N6O2S % C 59.13 H 6.14 N 19.70; found C 59.11 H 6.12 N 19.67
1-(1-((diphenylamino)methyl)-5-methoxy-2-oxoindolin-3-ylidene)-4-(3-methylpyridin-2-yl) thiosemicarbazide (4c)
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3372, 2867, 1702, 1620, 1292, 1075 cm-1 ; 1H NMR (DMSO d6): d 8.09 (m, H), 7.6 (d, H), 7.38 (d, H), 7.1 (d, H), 7.04 (m, 4H), 7.0 (s, H), 6.8 (t, H), 6.58 (m, 2H), 6.51 (d, H), 6.43 (m, 4H), 4.73 (s, 2H), 4.0 (s, H), 3.73 (s, 3H), 2.32 (s, 3H); MS (relative intensity): m/z value 522.18 (12); Calcd. for C29H26N6O2S % C 66.65 H 5.01 N 16.08; found C 66.64 H 5.00 N 16.05
1-(5-methoxy-2-oxo-1-(pyrrolidin-1-ylmethyl)indolin-3-ylidene)-4-(3-methylpyridin-2-yl) thiosemicarbazide (4d)
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3358, 2848, 1695, 1620, 1288, 1045 cm-1 ; 1H NMR (DMSO d6): d 8.09(m, H), 7.6 (d, H), 7.38 (d, H), 7.1 (d, H), 7.0(s, H), 6.8 (t, H), 6.51 (d, H), 4.03 (s, 2H), 4.0 (s, H), 3.73 (d, H), 2.32 (s, 3H), 2.25(m, 4H), 1.59 (m, 4H); MS (relative intensity): m/z value 424.17 (27); Calcd. for C21H24N6O2S % C 59.41 H 5.70 N 19.80; found C 59.39 H 5.67 N 19.78
1-(5-methoxy-2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene)-4-(3-methylpyridin-2-yl) thiosemicarbazide (4e)
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3352, 2843, 1692, 1627, 1286, 1037 cm-1 ; 1H NMR (DMSO d6): d 8.09 (m, H), 7.6 (d, H), 7.38 (d, H), 7.1 (d, H), 7.0 (s, H), 6.8(t, H), 6.51 (d, H), 4.03 (s, 2H), 4.0 (s, H), 3.73 (s, 3H), 2.32 (s, 3H), 2.24 (m, 4H), 1.50 (m, 6H); MS (relative intensity): m/z value 438.18 (32); Calcd. for C22H26N6O2S % C 60.25 H 5.98 N 19.16; found C 60.23 H 5.97 N 19.13
4-(5-chloropyridin-2-yl)-1-(1-((dimethyl amino)methyl)-5-methoxy-2-oxoindolin-3-ylidene) thiosemicarbazide 4f
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3365, 2851, 1679, 1617, 1289, 1086 620 cm-1 ; 1H NMR (DMSO d6): d 8.31(d, H), 7.68 (t, H) 7.6 (d, H), 7.1 (d, H), 7.0(s, H), 6.89 (d, H), 6.8 (t, H), 4.03 (s, 2H), 4.0 (s, H), 3.73 (s, 3H), 2.27 (d, 6H); MS (relative intensity): m/z value 418.1 (32); Calcd. for C18H19Cl N6O2S % C 51.61 H 4.57 N 20.06; found C 51.59 H 4.54 N 20.05
4-(5-chloropyridin-2-yl)-1-(1-((diethylamino) methyl)-5-methoxy-2-oxoindolin-3-ylidene) thiosemicarbazide (4g)
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3382 2856, 1683, 1637, 1281, 1078, 628 cm-1; 1H NMR (DMSO d6): d8.31(d, H), 7.68(t, H) 7.6 (d, H), 7.1 (d, H), 7.0(s, H), 6.89(d ,H), 6.8 (t, H), 4.03(s, 2H), 4.0 (s, H), 3.73(s, 3H), 2.40 (m, 4H), 1.00(m, 6H); MS (relative intensity): m/z value 446.13 (35); Calcd. for C20H23Cl N6O2S % C 53.74 H 5.19 N 18.80; found C 53.71 H 5.17 N 18.78
4-(5-chloro pyridin-2-yl)-1-(1-((diphenyl amino) methyl)-5-methoxy-2-oxoindolin-3-ylidene) thiosemicarbazide (4h)
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3374, 2854, 1693, 1632, 1284, 1058 and 624 cm-1; 1H NMR (DMSO d6): d 8.31 (d, H), 7.68 (t, H) 7.6 (d, H), 7.1 (d, H), 7.04 (m, 4H), 7.0 (s, H), 6.89 (d, H), 6.8 (t, H), 6.58 (m, 2H), 6.43(m, 4H), 4.73 (s, 2H), 4.0 (s, H), 3.71 (s, 3H); MS (relative intensity): m/z value 542.13 (21); Calcd. for C28H23Cl N6O2S % C 61.93 H 4.27 N 15.48; found C 61.91 H 4.25 N 15.46
4-(5-chloropyridin-2-yl)-1-(5-methoxy-2-oxo-1-(pyrrolidin-1-ylmethyl)indolin-3-ylidene) thiosemicarbazide (4i)
The sample was recrystallized using chloroform and petroleum ether. IR(KBr): 3388, 2850, 1682, 1615, 1280, 1023, 629 cm-1; 1H NMR (DMSO d6): d 8.31(d, H), 7.68 (t, H) 7.6 (d, H), 7.1 (d, H), 7.0 (s, H), 6.89 (d ,H), 6.8 (t, H), 4.03 (s, 2H), 4.0 (s, H), 3.74 (s, 3H) 2.25 (m, 4H), 1.59 (m, 4H) ; MS (relative intensity): m/z value 444.11 (16); Calcd. for C20H21Cl N6O2S % C 53.99 H 4.76 N 18.89; found C 53.97 H 4.73 N 18.87
4-(5-chloropyridin-2-yl)-1-(5-methoxy-2-oxo-1-(piperidin-1-ylmethyl)indolin-3-ylidene) thiosemicarbazide (4j)
The sample was recrystallized using chloroform and petroleum ether IR(KBr): 3390, 2851, 1692, 1634, 1292, 1071, 632 cm-1; 1H NMR (DMSO d6): d 8.31(d, H), 7.68 (t, H) 7.6 (d, H), 7.1 (d, H), 7.0 (s, H), 6.89 (d, H), 6.8 (t, H), 4.03 (s, 2H), 4.0 (s, H), 3.71 (s, 3H), 2.24 (m, 4H), 1.50 (m, 6H); MS (relative intensity): m/z value 458.13 (15); Calcd. for C21H23Cl N6O2S % C 54.96 H 5.05 N 18.31; found C 54.94 H 5.03 N 18.29
Analgesic activity
The analgesic activiy of isatin derivatives 4a-j were assessed by acetic acid induced writhing reflex in mice. Groups of 6 mice of either sex with body weight of 22 to 41 gm were used. Appropriate volume of acetic acid solution was administered to the first group (which serves as control), placed them individually under glass jar for observation.
Onset on wriths were noted, the number of abdominal contractions were recorded, trunk twist response and extension of hind limbs as well as the number of animals showing such response during a period of 10 minutes were also recorded.
To the second group of animals diclofenac sodium 45mg/kg were administered. Fifteen minutes later, to these animals acetic acid solution were administered. The onset and severity of writhing response were noted. The mean writhing scores in control and diclofenac sodium treated groups were calculated (Table 2).
Statistical analysis
Results are expressed as mean ±SEM. n represents the number of animals. Data obtained from pharmacological experiments were analyzed by one way (ANOVA) followed by Dunnet’s “t” test and used to evaluate the results, employing Pharmacologic Calculation System Version 4.1.
Results and Discussion
The structures, yields and melting points of the compounds are listed in Table 1. The Schiff bases of isatin derivatives were synthesized by condensation of the keto group of isatin with various thiosemicarbazides and further with secondary amines to get Mannich bases. All spectral data are in accordance with assumed structures. All the synthesized compounds were tested for analgesic activity. The synthesized compounds 4b, 4e, 4g and 4j show significant analgesic activity when compared to standard diclofenac sodium (45mg/kg).
Table 1: Structures, yields and melting points of the synthesized compounds.
| Compounds
|
R
Yield |
R1
|
R2
|
m.p | Crystallization solvents (1:1) |
| (% ) |
|
(°C) | |||
| 4a
|
CH3
68 |
|
H
|
160 | chloroform and petroleum ether |
| 4b
|
CH3
74 |
|
H
|
172 | chloroform and petroleum ether |
| 4c
|
CH3
71 |
|
H
|
182 | chloroform and petroleum ether |
| 4d
|
CH3
78 |
|
H
|
164 | chloroform and petroleum ether |
| 4e
|
CH3
81 |
|
H
|
150 | chloroform and petroleum ether |
| 4f
|
71 |
H
|
Cl
|
162 | chloroform and petroleum ether |
| 4g
|
77 |
H
|
Cl
|
166 | chloroform and petroleum ether |
| 4h
|
69 |
H
|
Cl
|
170 | chloroform and petroleum ether |
| 4i
|
73 |
H
|
Cl
|
168 | chloroform and petroleum ether |
| 4j
|
80 |
H
|
Cl
|
158 | chloroform and petroleum ether |
Table 2 : Analgesic effect of synthesized compound by writhing reflex method.
| Treatment | Dose (mg/kg) | Mean writhing
± S.E.M |
Percentage
protection |
| Control | Saline | 32±0.7303 | – |
| Diclofenac
sodium |
45mg/kg | 8.3±0.33** | 73.96 |
| 4a | 100mg/kg | 30.8±0.4773NS | 6.25 |
| 4b | 100mg/kg | 26.5±0.4282** | 17.18 |
| 4c | 100mg/kg | 30±0.730NS | 6.25 |
| 4d | 100mg/kg | 27.3±0.494* | 14.59 |
| 4e | 100mg/kg | 26.6±0.4216** | 16.58 |
| 4f | 100mg/kg | 29.5±0.6708* | 7.8 |
| 4g | 100mg/kg | 26.8±0.307* | 16.25 |
| 4h | 100mg/kg | 27±0.3651** | 15.62 |
| 4i | 100mg/kg | 27.81±0.3073* | 13.09 |
| 4j | 100mg/kg | 26.16±0.3073** | 18.2 |
**P<0.01 by Dunnet ‘t’ test (multiple comparison test) compared with control.
Values are expressed in mean ± SEM (n = 6)
Conclusion
The synthesized compounds shows significant analgesic activity, but generally, the synthesized compounds 4b, 4e, 4g and 4j (P<0.01) possess remarkable analgesic activity. Therefore, they seem to be really promising compounds for their analgesic activity. The synthetic studies should be continued concerning this group of compounds followed with further in vivo studies.
Acknowledgements
The authors are thankful to Vel’s College of Pharmacy and its management for providing research facilities and encouragement and to our friends those who helped us to complete this research.
References
- Bhattacharya, S.K and Chakrabarti, S. “Dose- related proconvulsant and anticonvulsant activity of isatin, a putative biological factor, in rats.” Indian J. Exp. Biol., 36: 118-121 (1998).
- Pajouhesh, H., Parson, R. and Popp, F. D. “Potential anticonvulsants VI: Condensation of isatin with cyclohexanone and other cyclic ketones.” J. Pharm. Sci., 72: 318-321(1983).
- Bhattacharya, S. K. “Anticonvulsant activity of intraventricularly administered atrial natriuretic peptide and its nhibition by isatin,” Biog.Amines, 14: 131-141 (1988).
- Pandeya, S. N. and Sriram, D. “Synthesis and screening of antibacterial activity of Schiff and Mannich bases of isatin derivatives,” Acta. Pharm. Turc. 40: 33 (1998).
- Pandeya, S.N., Sriram, D., Nath, G. and De Clercq, E. “Synthesis, antibacterial, antifungal and anti-HIV activity of Schiff and Mannich bases of isatin with N-(6-chlorobenzthiazol-2-yl) thiosemicarbazide,” Indian J. Pharm Sci. 61: 358-361 (1999).
- Varma, R.S. and Nobles, W.L. “Substituted N-amino methyl isatins,” J. Med. Chem. 10: 972-974 (1967).
- Pandeya, S.N., Yogeeswari, P., Sriram, D., De Clercq, E., Pannecouque, C. and Witvrouw, M. “Synthesis and screening for anti-HIV activity of some N-Mannich bases of isatin derivatives,” Chemotherapy 45:
192-196 (1999). - Pandeya, S.N., Sriram, D., Nath, G. and De Clercq, E. “Synthesis, antibacterial, antifungal and anti-HIV activities of norfloxacin Mannich bases,” Eur. J. Med. Chem. 35: 249-255 (2000).
- Pandeya, S.N., Sriram, D., Nath, G. and De Clercq, E. “Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin and its derivatives with triazole,” Arzneimittel-Forschun /Drug Res. 50: 55-59 (2000).
- Sarangapani, M. and Reddy, V.M. “Pharmacological screening of isatin-N-(2-alkyl benzoxazole-5-carbonyl) hydrazones,” Indian J. Pharm. Sci. 59: 105 (1997).
- Cesur, N. and Cesur, Z. “Synthesis of some 4-thiazoline and 4H-1,2,4- triazole derivatives of imidazo(1,2-a) pyridine as possible anticonvulsants.” Farmaco 49: 679-681 (1994).
- Phillips, O.A and Knaus, E. E. “Synthesis and anticonvulsant activity of 3-(3’- trifluoromethyl phenoxy)-pyridines and dihydropyridines.” Drug Des Deliv. 7: 279-286 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





