How to Cite | Publication History | PlumX Article Matrix
Synthesis and biological activities of a novel series of indazole derivatives
M. V. Aanandhi1*, A. A. Joseph1, R. ChandraKumar1, M. Koilraj1, R. Sujatha2 and P. Shanmugasundaram1
1Vel’s College of Pharmacy. Chennai - 600 117 India.
2Government Arts and Science College for Women, Burgur - 635 104 India.
Corresponding Author E-mail: mvaanandhi@yahoo.co.in
ABSTRACT: The newly synthesized indazole derivatives were evaluated for analgesic, anti-inflammatory and antipyretic activities. The synthesized compounds have been characterized on the basis of IR, NMR, mass and elemental analysis. Compound (b) showed significant analgesic, anti-inflammatory and antipyretic activities at the dose level of 500mg/kg when compared to standard.
KEYWORDS: Indazole; analgesic; anti-inflammatory; antipyretic
Download this article as:| Copy the following to cite this article: Aanandhi M. V, Joseph A. A, ChandraKumar R, Koilraj M, Sujatha R, Shanmugasundaram P. Synthesis and biological activities of a novel series of indazole derivatives. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Aanandhi M. V, Joseph A. A, ChandraKumar R, Koilraj M, Sujatha R, Shanmugasundaram P. Synthesis and biological activities of a novel series of indazole derivatives. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6759 |
Introduction
Indazole derivatives were found to exhibit many biological activities such as anti-inflammatory[1,2], antifungal, antimicrobial[3] activities and also used as adjuvant in radiation therapy [4]. Some indazole analogues were used as novel antiplatelet agents [5]. The reports of all these activities of indazole derivatives prompted us to take up the study in detail. The research work has been carried out by comprising indazole moiety for anti-inflammatory, antipyretic and analgesic activities. The synthesized compounds were taken for pharmacological activities.
Materials and Methods
Synthesis of 3-methyl-1-H-indazole (a)
O-hydroxy acetophenone (0.005 mole), 85% hydrazine hydrate (0.010) and glacial acetic acid (10 drops) were placed in a conical flask and the mixture was stirred at 110-112o c for about 25 min. after cooling polyphosphoric acid was added to the mixture. The product was extracted with ethyl acetate and the combined extracts were washed with water, dried on anhydrous sodium sulphate, evaporated and was recrystallized from methanol. (IR 3020(Aromatic),3387(N-H),2963(alkyl),1640(C=N),NMR2.79(s,3H),7.20(d,1H),7.34(m,1H),7.60(m,1H),7.85(t,1H),12.4(s,1H), MS 132.07 C 72.70,H 6.10, N 21.20, Yield – 83%)
Synthesis of 3-methyl-1-carbethoxy ethyl indazole (b)
A mixture of 3-methyl-1H-indazole (0.01 mole), ethyl chloro acetate (0.01 mole), anhydrous acetone (60 ml) and anhydrous potassium carbonate (0.02 mole) was heated under reflux for 24 hour. After cooling, it was filtered, washed with acetone and the filtrate evaporated. The solid mass thus obtained was recrystallized from methanol. (IR3028(Aromatic),3380(NH),2968(alkyl),1642(C=N),1728(C=)NMR1.06(m,3H),2.49(m,2H),2.76(s,3H),4.89(s,2H),7.22(d,1H), 7.36(m,1H), 7.61(m,1H), 7.85(t,1H) MS 202.11 C 71.26,H 6.98,N 13.85, Yield – 80%).
Synthesis of 3-methyl-1-carbethoxy methyl indazole (c)
A mixture of 3-methyl-1-carbethoxy indazole (0.005 mole) and sodium hydroxide solution (20 ml) was refluxed on a heating mantle for 3 hrs. The mixture was then cooled, filtered and the filtrate acidified with 10% sulphuric acid. The resultant precipitate was filtered ,washed with water and was recrystallized from methanol.(IR 3021(Aromatic),3376(N-H),2960(alkyl),1641(C=N),1723(C=) NMR 2.09(s,3H),2.74(s,3H),4.87(s,2H),7.23(d,1H), 7.37(m,1H),7.62(m,1H), 7.87(t,1H) MS 188.09 C 70.19,H 6.43,N 14.88 Yield – 82%).
Wistar strain albino rats of either sex weighing 200-250 gm, were kept in the department animal house and maintained under standard environmental conditions and was fed with standard pellet diet and water ad libtum . The experiments were performed followed by approval from Institutional Animal Ethical Committee.
Acute toxicity studies
Acute toxicity studies were carried out following OECD guidelines and were found to be safe upto 500mg/kg body weight in albino wistar rats.
Analgesic activity
Hot plate method [6]
Wistar albino rats were placed on a hot plate maintained at a temperature of 55+0.5oc. The reaction time was recorded, when the animals licked their fore and hind paws or jumped at 30, 60 and 90 minutes. Animals that showed a reaction time of less than 10 seconds at 0 minute were discarded and the selected animals were divided into four groups of six animals each. The animals in groups received the schedule of treatment as mentioned above.
Writhing test: Acetic acid induced writhing assay [7]
0.25 ml of 1% acetic acid solution was administered intra peritoneally to produce writhing in mice. The severity of pain response (writhing) was assessed by counting the number of wriths (constriction of abdomen, turning of trunk (twist) and extension of hind limbs) in mice. Number of wriths per animal was counted during a 30 minute session beginning 3 minutes after injection of acetic acid. The protective role of synthesized compounds on acetic acid induced writhing was evaluated.
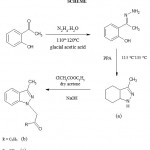 |
Scheme 1
|
Tail clip method [8]
Drugs were administered to all groups as described in the treatment protocol. 30 minutes later, a tail clip applied at the base of the tail of rats and they were observed for reaction of pain. The time at which animals tried to remove the clip was taken as the cut off time.
Anti-inflammatory activity
Carragennan induced rat paw edema [9]
Edema was induced by sub plantar injection of 0.1 ml of freshly prepared suspension of carragennin into the left hind paw of rats. The volume of injected and contra lateral paws were measured 1, 2, 3 and 4 hr after induction of inflammation using a plethysmometer. All the treatments were given 30 minutes prior to the injection of carragennin as per the treatment protocol.
Anti-pyretic activity [10]
Rats selected for the study was fastened overnight allowing water ad libitum. Initial rectal temperature was recorded using Hick’s clinical thermometer. Pyrexia was induced by subcutaneous injection of TAB vaccine 1 ml/kg body weight. Six hours later pyrexia was assessed and those animals that did not show a minimum rise of 1.5oF were discarded. The animals thus found fit for the study was divided into four groups as described above in the treatment protocol and drugs were administered. Pyrexia was recorded at hourly intervals for 4 hours after drug administration.
Results and Discussion
All the compounds were characterized on the basis of physical data, elemental analysis,IR ,NMR and mass spectra. NMR of each compound was recorded in CDCl3 and characterized on the basis of a specific peak in ppm using TMS as an internal standard. Characteristic IR bands are recorded using KBr pellets.
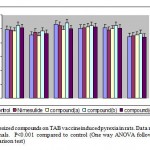 |
Figure 1
|
Analgesic activity [11]
In acetic acid induced writhing test, compound (b) at the dose level of 250 and 500 mg/kg/p.o significantly inhibited writhing (p<0.01 and p<0.001) respectively compared to control. Acetic acid induced writhing involves the release of arachidonic acid metabolites via cyclooxygenase pathway and prostaglandin biosynthesis [11]. Acetic acid induced writhing in mice is due to increase in peritoneal fluid levels of PGE2 and PGF2α [13]. Compound (b) might be exerting its analgesic action by means of cyclooxygenase pathway inhibition of prostaglandins. Compound (b) at the dose of 250 and 500 mg/kg significantly increased (p<0.001) the latency time of rats exposed to hot plate and tail clip.
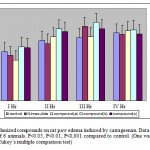 |
Figure 2
|
Anti-inflammatory activity
Carrageenan induced inflammation [12]
Carrageenan induced inflammation is a widely used acute inflammatory model to evaluate the anti-inflammatory activity of drugs. Carrageenan is a monopolysaccharide derived from Irish Sea moss, chondrus. Carragennin induced inflammation involves three distinct phases. Histamine and serotonin are released in the first phase, kinins are released in second phase and prostaglandins are released in the third phase [12]. Compound (b) at the dose level of 500 mg/kg caused a significant reduction in rat paw edema only during the third hour, whereas it didn’t produce any significant inhibition during the first and second hours. Compound (b) at the dose level of 250 mg/kg was ineffective in reducing the inflammation. The probable mechanism of anti-inflammatory action of compound (b) may be due to its interference in the cyclooxygenase pathway rather than the lipooxygenase pathway, since it is interfering with prostaglandin biosynthesis as evidenced by the maximum anti-inflammatory activity at the end of 3rd hour after the challenge with carrageenan.
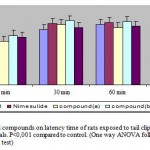 |
Figure 3
|
Antipyretic activity [13]
Compound (b) at the dose of 500 mg/kg exhibited significant antipyretic activity at the 2nd and 3rd hour of TAB vaccine induced pyrexia. But compound (b) at the dose of 250 mg/kg produced significant antipyretic activity only at the 3rd hour. The role of PGE2 in pyrexia is postulated to increase the set point of the hypothalamic thermostat to a higher level leading to increased heat production and decreased heat loss. Fever inducing effects of endogenous pyrogens are mediated via increase in hypothalamus PGE2 activity. Hence the antipyretic activity of compound (b) in TAB vaccine induced pyrexia may be attributed to its inhibition of PGE2 biosynthesis at hypothalamic level.
Acknowledgement
The authors are thankful to Dr.Ishari K.Ganesh, Chairman, Vel’s college of pharmacy, Chennai, for his encouragement and support for this study.
References
- Handy RL, Moore PK, Effect of 7-nitroindazole on carrageenan induced hind paw edema and its activity. Br.J.Pharmacol 123, 1119-26, 1998.
- Scindler R, Fleishhauer I, Hofgen N, Sauer W, Egerland U, Poppe H, Heer S, Szelenyi I, Kutscher B, Engel J. 1,5 disubstituted indazol-3-ols with anti-inflammatory activity, Arch.Pharm 3311, 13-21, 1998.
- Raj AA, Raghunathan R, Sridevi Kumari Mr, Raman N. Synthesis, antimicrobial and anti fungal activity of a new class of spiropyrrolidines. Bio Org MedChem 11, 407-19, 2003.
- Dassonnevitle L, Bailly C. Stimulation of topoisomerase II mediated DNA cleavage by an indazole analogue of lucanthone. Biochem Pharmacol, 58, 1307-12, 1999.
- Lee FY, Lien JC, Juang LJ, Huang TM, Tasai SC, Teng CM, Wu CC, Cheng FC ,Kuo SC. Synthesis of 1-benzyl-3-(5’-hydroxymethyl-2’furyl)indazole analogues as novel antiplatelet agents. J.Med.Chem, 44, 3746-9, 2001.
- Asparicia L. Some aspects of pharmacognosy of butibufen, a non steroidal anti-inflammatory agent. Arch.Int.Pharmacodyn., 227:130-141, 1977
- Koster R, Anderson M and De Beer EJ. Acetic acid for analgesic screening. Fed. Proc., 18:412-416, 1959.
- Sing GB and Atal CK. Pharmacology of an extract of salai guggal exn Boswellia Serrata, a new non steroidal anti-inflammatory agent. Agents and Action, 18:407-412, 1984.
- Winter CA, Risley EA AND Nuss GW, Carrageenin- induced edema in hind paw of rat as an assay for anti-inflammatory drugs. Proc.soc.Exp.Biol.Med., 11:544-547, 1962.
- Pendse VK, Dadich AP, Mathur PN, Bal MS and Madan BR. Ind.J.Pharmacol., 9:221, 1977.
- Duarte DG, Nakmura M (Ferreira). Participation of sympathetic system in acetic acid induced writhing in mice. Braz.J.Med., 21:341-343, 1988.
- Di Rosa M and Sorrentino L. The mechanism of the inflammatory effect of carrageenan Eur.J.Pharm., 4:340-342, 1968.
- Deraedt R, Jougney S, Delevalee F and Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur.J.Pharmacol., 61:17-24, 1980.

This work is licensed under a Creative Commons Attribution 4.0 International License.





