How to Cite | Publication History | PlumX Article Matrix
Tannase enzyme: The most promising biocatalyst for food processing industries
Kannan Natarajan, Aravindan Rajendran* And Viruthagiri Thangavelu
Biochemical Engineering Laboratory, Department of Chemical Engineering, Annamalai University, Annamalai nagar - 608 002 India.
Corresponding Author E-mail: donaravind@yahoo.com
ABSTRACT: Tannase (tannin acyl hydrolase, E.C.3.1.1.20) is an extracellular hydrolase enzyme that catalyzes the hydrolysis of ester and depside bonds in hydrolysable tannins or gallic acid esters, liberating glucose and gallic acid (GA). Tannase cleaves the ester linkages between galloyl groups present in various compounds such as epigallocatechin and epigallocatechin gallate that are present in green tea leaves. The enzyme could be obtained from many sources starting from prokaryotes to higher eukaryotes. Vital minutiae such as regulation pathways, catalytic characteristics and other properties remain unrevealed which limits its usage in large scale. This study essentially elicits the information on tannase substrates, mechanism, applications, and the recent trends in the purification of tannase.
KEYWORDS: Tannase; gallic acid; Biocatalyst; Food processing industries
Download this article as:| Copy the following to cite this article: Natarajan K, Rajendran A, Thangavelu V. Tannase enzyme: The most promising biocatalyst for food processing industries. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Natarajan K, Rajendran A, Thangavelu V. Tannase enzyme: The most promising biocatalyst for food processing industries. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6611 |
Introduction
Tannin is the fourth most abundant plant constituent after cellulose, hemicellulose and lignin.1 Tannins are water soluble poly-phenols with varying molecular weight depending on the bonds possessed with proteins and polysaccharides. They are astringent and also possess good antioxidant property. Tannins are an important ingredient in the process of tanning leather that prevents decomposition and ususlaly imparts colour to the leather. Tannins bind to the protein molecules and precipitates them, creating problems in the purification and clarification in the distilleries and tea industries. It inhibits the absorption of iron, calcium and zinc from the food, when tea is consumed along with food. Tannins are quite resistant to microbial attack and are known to inhibit the growth of some microorganisms. Biodegradation of soil organic matter is usually a very complex process that involves the degradation of organic matter by microorganisms to utilize the constituents such as carbon, nitrogen. The large amounts of polyphenolic compounds on the tannin substrate structure can form complexes with the extra and intracellular enzymes from the biodegradative organisms. This complication leads to inhibition of the biodegradative enzymes which in turn leads to a loss in the microbial growth and eventually an increase in the bioconversion time taken for the decomposition of soil organic matter.
However, tannase has been found fortuitously when Van Tieghem, 1867 studied the production of gallic acid from the aqueous solution of tannin. Tannase hydrolyses the tannic acid or tannins in to gallic acid and glucose by hydrolyzing the ester bonds in the tannin.2 By degrading the tannin, the toxicity in the tannery effluents could be reduced and it can also control the level of tannin in tea and distilleries. Utilization of such an active enzyme in the industries could reduce the problems due to tannins. The utilization of the enzyme in the large scale is limited, due to the unfurnished details about their properties, mechanisms and the cost of production for a pure enzyme product.
Sources of Tannase
Tannase can be obtained from both eukaryotic and prokaryotic sources namely bacteria, fungus, plants, certain species of insects and cattle.3 Among the plants, the vegetables, fruits, leaves and branches with very high content of tannin produce a considerable amount of tannase and it is said that tannase is an important enzyme required for the formation of gallic acid in plants. The production of the enzyme has been reported from the Oak tree.4The enzyme has also been isolated from the bovine intestine, from the ruminal mucous and in gall larva. But the most important source for the production of enzyme tannase is the microbial source. Microbial tannase is more stable than tannase from other sources like plants or animals. Tannase is produced by a number of microorganisms like fungi (Aspergillus, Penicillium, Rhizopus sp.), yeast (Candida sp.) and bacteria (Bacillus species).
Microbial Tannase
Many Bacterial species have found to produce both the intracellular and extracellular tannase enzyme which can hydrolyze natural tannic acid very efficiently. It has been showed that strains of Bacillus pumilus, B. polymyxia, and Klebsiella lanticola were able to produce extracellular tannase with chestnut bark as the sole source of carbon. The most abundant group of bacteria that were able to degrade tannins is found in the gastrointestinal track of ruminants.5Lactobacillus plantarum was found to produce appreciable amount of tannase. Achromobacter sp., Pseudomonas solanaceaum, Selenomonas ruminatium were found to be moderate producers of tannase enzyme.6Tannase is produced as a membrane bound enzyme in most of the organisms and not all tannase is equally active against the different tannin substrates. Fungal tannases have a better activity in degrading hydrolysable tannins, whereas yeast tannases degrade tannin better and has a lower affinity for naturally occurring tannins.5 Among the fungal species the best producer of tannase were found to be Aspergillus oryzae, A. niger, A. japonicus, A. awamori, whereas the moderate producers were A. flavus, Penicillium species, Trichoderma sp., Helicostylum sp., and Cunnighamella sp.6Tannin degradation by yeasts has not been studied to its full potential. Aoki et al.,7 1976 isolated and reported the enzymatic degradation of gallotannins by yeast species belonging to Candida that was able to produce tannase. The tannase from this yeast was able to hydrolyze the ester and depside linkages from tannic acid. Production of tannase has been noted in Candida sp., Pichia sp., Debaryomyces hansenii, Mycotorula japonica.8,9and Saccharomyces cerevisae .10
Table 1: Important microbial sources of Tannase.
| Type of Microorganism | Microorganism | Total tannase activity
|
Reference
|
| Fungus | A. alliaceus | 0.17 (U/g ) | [13]
|
| A. fumigatus | 1.04 (U/g ) | ||
| A. niger | 1.22 (U/g ) | ||
| A. oryzae | 1.03 (U/g ) | ||
| A. niger (MTCC 2425) | 9.7 (U/mL ) | [14] | |
| A.flavus | 1.1 (U/mL) | ||
| Aspergillus niger (MS101) | 3.6 (U/mL) | ||
| Fusarium sp. | 1.8 (U/mL ) | ||
| Penicillium sp. | 0.1 (U/mL) | ||
| Trichoderma sp. | 3.0 (U/mL) | ||
| Rhizopus oryzae | 6.12 (U/mL) | [15] | |
| Penicillium notatum | NS | [16] | |
| Bacteria | Bacillus licheniformis | 0.21 (U/mL) | [17]
|
| Bacillus cereus (KBR 9) | 0.22 (U/mL) | ||
| Lactobacillus sp. | 085 (U/g ) | [18] | |
| Lactobacillus plantarum | 6.0 (U/mL) | [19] | |
| L. plantarum(ATCC 14917) | 5.7 ± .2(mU/mL) | [20] | |
| L. plantarum ( CNRZ 184) | 0.8 ± .1(mU/mL) | ||
| L. plantarum (61D) | < 0.1(mU/mL) | ||
| L . koalarum (ACM 3666) | 0.7 ± 0.2(mU/mL) | ||
| S.gallolyticus(ACM 3611) | 2.8 ±(mU/mL) | ||
| Yeasts
|
Candida sp. | NS | [7] |
| Pichia sp. | NS | [8] | |
| Debaryomyces hansenii
|
NS | [8] | |
| Plant | Quercus rubra | NS | [4] |
| Terminalia chebula | NS | [3, 4] | |
| Caesalpinia coriaria | |||
| Quercus robur | |||
| Rhus typhina |
Plant Tannase
Many tannin rich plant materials have been identified that contain tannase activity, for example Terminalia chebula (Myrobolan fruits), Caesalpinia coriaria (divi-divi pods) and from Quercus robur (English oak) and from the Rhus typhina (leaves of the Karee tree).3,4 Cell free extracts from the varieties of plants gained ability to hydrolyze the substrate β-glucogallin (1-O-galloyl-β-D glucopyranose) in vitro assays, due to the presence of the enzyme tannase.
The plants and microorganisms have the ability to overcome all the degradative resistance of tannins and in return utilize them in their metabolism. Plants during growth synthesize large amounts of various compounds that are necessary for the fruit production that requires gallic acid, chebulinic acid and hexahydroxyphenic acid, and as the fruit ripens, these acids might become esterified with glucose with the help of tannase to form complex tannins. Upon abscission of the fruit the esterase activity in the tannase may contribute to the hydrolysis of the preformed tannins. When the plant leaves are attacked by herbivores the cells lose compartmentation, which brings the tannase into contact with the tannin substrate in the leaves. The substrate is then hydrolyzed into harmful low molecular weight phenolic degradative compounds, which can be precursors for toxic substances in higher plants.
Properties of Tannase
Fungal tannase possess good activity at an optimum pH range of about 5.0-7.0 and the stability of the enzyme could be observed at an wide range of pH of about 3.5-8.0 with an optimal temperature range of 30-60ºC for different genus of fungus, whereas the bacterial tannase has an optimum activity at a pH range of 4.5-5.5 with an optimal temperature range of 30-40ºC. The plant tannase isolated from penduculate oak was shown to be active over a wide pH range with an optimum of approximately 5.0 at a temperature of 35ºC and 40ºC.
Limited molecular analysis has been made in fungal tannase when compared to bacterial tannase. The molecular weight of tannase from Aspergillus strains, ranges between 150,000 Da to 350,000 Da,9 whereas in the plant pendunculate oak it was about 300,000 Da.4 The gene sequence of Aspergillus oryzae codes for 588 amino acids with no introns and the tannase produced has a molecular weight of 64,000 Da.11Tannase from A. oryzae has four pairs of two subunits with one subunit having molecular weight of 30,000 Da and the other with 33,000 Da that are linked by disulfide bond, forming a hetero-octamer with a molecular weight of about 300,000 Da. Tannase from Candida sp. K1 also contains two subunits of 120,000 Da each that could be separated after treatment with SDS and 2.mercaptoethanol.7
Tannins as Substrate for Tannase
Tannins are naturally occurring polyphenolic compounds with varying molecular weights that occur naturally in the plant kingdom. These phenolic compounds differ from others by having the ability to precipitate proteins from solutions. In the plants these tannins are found in leaves, bark and wood. Tannins are considered to be the plant’s secondary metabolic products because they play no direct role in the plants metabolism. After lignin, tannins are the second most abundant group of plant phenolics. The large amount of phenolic hydroxyl groups allows the tannins to form complexes with proteins and to a lesser extent with other macromolecules like cellulose and pectin.12Tannins can be divided into two hydrolysable tannins and condensed tannins on the basis of their structure and properties.
Hydrolysable Tannins
Hydrolysable tannins are polyphenolic plant constituents derived from mono-to pentagalloyllated β-D-glucopyranose. These “simple esters” from the gallotannin subclass are extended by attachment of additional galloyl residues to the phenolic galloyl-OH groups to yield metadepsidic side chains of variable length. The allagitannin subclass is characterized by oxidative linkages of spatially adjacent galloyl residues of the core unit with the formation of hexahydroxydiphenoyl bridges.4
Condensed Tannins
Condensed tannins are also known as proanthocyanidins, and consist of phenols of the flavon type flavonoids. They are also called flavolans because they are polymers of flavan-3-ols such as catechin or flavan-3, 4-diols known as leucocyanidins. A very interesting difference between condensed tannins and hydrolysable tannins is the fact that condensed tannins do not contain any sugar moieties. An intermediate group also exists that combines both characteristics of hydrolysable tannins and condensed tannins. This family of tannins is called the catechin tannins. The catechin tannins are most abundant in tea leaves.21 Table 2 summarizes the different types of natural occurring tannins that can serve as substrates for tannase.22, 23
Table 2: Types of Tannins.
| Hydrolysable Tannins | Complex Tannins | Condensed Tannins |
| Tannins that are easily hydrolysable that forms glucose or quinnic acid.
Types: Gallic acid, Ellagic acid, Digallic acid and Chebulic acid |
It is a complex of Gallic or Ellagic acid with Catechins and Glucosides. | Polymeric roanthocyanidins – Complex made up of flavanoids and is not easily hydrolysable and are not degraded by Classical Tannases.
Types: Catechins, Quercetin |
| 1. Gallotannin – yields gallic acid or digallic acid or chebulic acid and glucose. These are the tannins degraded in a better way by tannases.
2. Ellagitannin – yields poly ellagic acid and glucose or quinnic acid on hydrolysis. Selective hydrolysis of the galloyl groups of the ellagitannin are performed by tannases. |
Catechin (falvon – 3 –ols) Has Cyanidin and Delphinidin.
Quercetin (Flavanols) (Both are degraded to Phloroglucinol and finally yield β-Ketoadipate, which could not be hydrolyzed further). |
Mechanism of Tannase Catalyzed Reactions
Even though tannic acid degrades the protein molecules (enzymes), the tannase hydrolyses the tannic acid very effectively.24 In plants tannase helps in the cell wall degradation by breaking some of the cross linking bonds that exist between the cell wall polymers.25 Tannase was shown to hydrolyze the esters of tannic acid completely to gallic acid and glucose with 2,3,4,6, -tetragalloyl glucose and two kinds of monogalloyl glucose as intermediates. This is supported by the facts that the same products were detected in the hydrolysate of 1, 2, 3, 4, 6, – pentagalloyl glucose, and that depsidic gallic acid of methyl-m-digallate was liberated first, where R1 and R2 are gallate and digallate respectively (Figure 1).
Figure 1
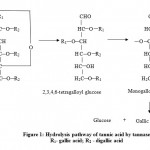 |
Figure 1: Hydrolytic Pathway of tannic acid by tannase (Albertse et al., 2002).
|
For a true enzyme-substrate complex to form the following
There should not be any restriction on the structure of an alcohol composing a substrate ester, although the acid should be gallic acid.
Any phenolic hydroxyl might react with the binding site of the enzyme and prevent the enzyme from forming a true ES-complex.
An ester bond or carboxyl does not link to the enzyme by itself, because an ester or carboxylic compound is not hydrolyzed by or inhibits the enzyme unless it has phenolic hydroxyls.24
Purification of Tannase
The extraction and purification of tannase depends on the enzyme location and the mode of fermentation (Submerged / Solid State Fermentation). The extraction of the intracellular tannase involves the addition of detergent molecules, citrate and phosphate buffers of concentration 0.05 M for pH maintenance. The purification of tannase was started in the 70`s and later many purification strategies were developed. Three steps were performed initially for the purification of Tannase. In the first step acetone was used for the precipitation at pH 4.6 followed by filtration to remove the precipitates and then the chromatographic separation to fractionate the pure enzyme.26
The process of purification involves the following steps:
Proteins in the extract were first removed by precipitation with ammonium sulphate at 50% (w/v) saturation. The 50% saturation of the extract precipitates the non-enzymatic proteins leaving out the tannase.
After precipitation the precipitate were discarded and the supernatant were precipitated at 80% saturation.
The enzyme precipitate at 80% collected by centrifugation was dissolved in 0.02 M acetate buffer (pH 4.7) and dialyzed overnight against distilled water.
The enzyme was then re-precipitated with acetone (50% v/v) under constant stirring for 30 min and the precipitate was separated by centrifugation making the enzyme free from acetone and then suspended in 0.02 M acetate buffer (pH 4.7) containing 20% (v/v) glycerol.27
For tannase extraction pestle and mortar methods was seemed to be better suited than the modern homogenizers and provided higher yield of the enzyme. In this method detergents like Triton X-100 was used which provided good yield. Use of Cetyl-Trimethyl Ammonium Bromide (CTAB) provided good yield but lesser than obtained with Triton X-100.28 The buffers citrate and phosphate were used and the optimum pH for the extraction was found to be 6.0. The use of Sephadex G-150 column chromatography for tannase purification followed by lyophilization to a minimum volume and applied on DEAE-Sephadex A-50 column yields high tannase activity.
The recombinant tannase was purified to homogeneticity from cultured broth supernatants by a simple procedure on DEAE-Sepharose fast flow chromatography and this procedure is highly efficient in providing large amounts of pure enzyme. Approximately 72 mg of pure recombinant tannase was obtained from a litre of crude extract and the specific activity of purified enzyme could reach 50,000 IU/g.10 At present a two step well-organized and trustworthy procedure for purification of P. variable tannase was developed. First step is the process of ultra-filtration of the culture filtrate after concentrating it to one-tenth of the original volume by using 100 kDa membrane that resulted in the yield of about 97% and 5.0 fold purification. It is followed by the gel-filtration chromatography in which the ultra filtered concentrate was purified to homogeneity using sephadex G-200 that results in 91% enzyme yield with 135 fold purification.29A similar procedure was reported for the separation of tannase from Aspergillus niger LCF 8 using the 200 kDa membrane.
Table 3: Summary of purification of tannase from various microorganisms.
| Organism | Extraction and Purification Method | Specific activity | Reference |
| Aspergillus nigerLCF 8 | Mechanic Hydrolysis of mycelia followed by Ultrafiltration | 1010 nkat/mg protein | [30] |
| Aspergillus niger LCF 8 | Enzyme hydrolysis of mycelia followed by Reverse Micellar system | 1090 nkat/mg protein | |
| Aspergillus japonicus | Extracellular enzyme extracted using PEG and Tannic acid for precipitation
|
91.89 U/mg 643.12 U/mg (finally) |
[31]
|
| Aspergillus niger MTCC 2425 | Crude extract isolated, dialysed and freeze dried. Reconstituted with Chromatography Sephadex G-150 followed by DEAE Sephadex A-50 | 355.6 U/mg protein | [28] |
| Aspergillus awamori nakazawa | Acetone precipitated fraction of extracellular tannase, run through Gel Filtration 250 column (with pore size 4µm) with the solvent 0.2 M acetate buffer | 3.23 U/ml (total activity) | [32] |
| Penicillium variable | Ultra filtration, followed by Gel Filtration G-200 | 2055 U/mg | [29] |
Applications of Tannase
Pharmaceutical Industry
An important application of Tannin Acyl Hydrolase (TAH) is the production of gallic acid from plant byproducts rich in tannins.33-35 Gallic acid is used as a synthetic intermediate for the production of pyrogallols and gallic acid esters.36 Today gallic acid is mainly used for the synthesis of trimethoprim (antibacterial agent), as well as for the production and synthesis of propyl gallate, which is used as an antioxidant in fats and oils.37 The gallic acid and propylgallate were in use as antioxidants in foods, cosmetics, hair products, adhesives, and lubricant industry.38-40 Weetal and Detar,37 1985 explored the possibility of its preparation by esterification of gallic acid by tannase. Tannase under low water conditions can catalyze the hydrolytic reaction as shown in figure 2. By employing biotechnological means to synthesize gallic acid huge expenses can be saved with better and more selective yields.
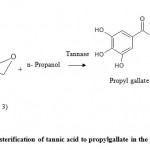 |
Figure 2: Transesterification of tannic acid to propylgallate in the presence of n-propanol using tannase.
|
Cold Tea Manufacture
Tannins in the tea leaf extracts could obstruct the absorption of ferrous, calcium ions from food when consumed along with food. In such conditions the utilization of tannase in the extract could convert all the tannins and have the ability to reduce the toxicity. Tea cream is formed when the tea is stored at or below temperatures of 4°C that prevents the formation of a product with high cold water solubility, which is a very large problem in the manufacturing of instant tea. Tannase has the catalytic activity to remove gallic acid moieties from tannins and the polyphenols from tea extract, resulting in cold water-soluble products. The treatment of tea with tannase enhances the natural levels of epicatechin and gallic acid, which in turn favours the formation of epitheaflavic acid, which is responsible for the bright reddish colour of tea. The reaction that follows is a deesterification between galloyl groups and various compounds in unconverted tea leaves.
Other Industries
Tannase is used as a clarifying agent in the beer and wine industries to remove the haze formation. In the tanneries that work with very high level of tannin and many poly-phenols, tannase is applied for the removal of such compounds from the effluent making it safer to the environment and also helps in the synthesis of gallic acid esters that finds applications as an antioxidant in the formation of propyl gallate used as an staining agent for the leather materials.41 Tannase is used in the manufacture of ordinary writing inks and dyes, as photographic developer; in the enzymatic synthesis of propyl gallate.15, 27, 42 It is also used in the preparation of animal feedings from sorghum that are made simpler without any tannin by the action of tannase. The anti oxidant activity of lentil flour has been increased by adding tannase to the flour.43 In addition it is also used as a sensitive analytical probe for determining the structure of naturally occurring gallic acid ester.44
Conclusion
Tannase possess immense ability to degrade complex tannins in to simpler compounds thereby decreasing the toxicity level in the tannery effluents an increasing the quality and the antioxidant content in the tea by removing the haze. Tannase could be produced in large scale industries by using fungal species, which are considered to be best producer. The tannase from various sources has diverse specificity towards substrate molecules and the development of tannase with good degree of specificity could help the future advancements. The cost of production for the enzyme should be reduced considerably by using solid state fermentation utilizing cheaper agro residues in the production medium and by optimizing the purification strategies. At the same time by understanding the mechanism of action of tannase and its properties could enhance the utilization of tannase in various fields.
References
- Swain T. Bonner J. and Varner J.E., Plant Biochemistry. Academic Press. New York, 552–580 (1965).
- Lekha P. and Lonsane B.K., Process. Biochem., 29, 497–503 (1994).
- Madhavakrishna W. Bose S. and Nayudamma Y., Bulletin Of Central. Leather. Research. Institute., 7, 1–11 (1960).
- Niehaus J.U. and Gross G.G., Phytochem., 45, 1555–1560 (1997).
- Deschamps A.M. Otuk G. and Lebeault J.M., Ferment. Technol., 61, 55–59 (1983).
- Bhat T.K. Singh B. and Sharma O.P., , 9, 343–357 (1998).
- Aoki K. Shinke R. and Nishira H., Biol. Chem., 2, 297–302 (1976).
- Deschamps A.M. and Lebeault J.M., Lett., 6, 237–242 (1984).
- Balmers R. Contreras-Esquivel J.C. Rodriguez-Herraera R. Ramirez Coronel A. and Aguilar C.N., Wissenschaftund. Technologies., 37, 857–864 (2004).
- Zhong X. Peng L. Zheng S. Sun Z. Ren Y. Dong M. and Xu A., Protein. Expr. Purif., 36, 165–169 (2004).
- Hatamoto O. Watari T. Kikuchi M. Mizusawa K. and Sekine H., , 175, 215–221 (1996).
- Mueller – Harvey I. Reed J.D. and Hartley L.D., Sci. Food. Agric., 39, 1–14 (1987).
- Albertse E.K., M.Sc. thesis, Faculty of Natural and Agricultural Sciences, Department of Microbiology and Biotechnology, University of the Free State Bloemfontein, South Africa (2002).
- Murugan K. Saravanababu S. and Arunachalam M., Bioresour. Technol., 98, 946–949 (2007).
- Hadi T.A. Banerjee R. and Bhattarcharyya B.C., Bioprocess. , 11, 239–243 (1994).
- Ganga P.S. Nandy C. and Santappa M., Leather. Sci., 24, 8–16 (1977).
- Mondal K. Banerjee D. Banerjee R. and Pati B., J. Gen. Appl. Microbiol., 47, 263–267 (2001).
- Sabu A. Augur C. Swati C. and Pandey A., Process. Biochem., 41, 575–580 (2006).
- Ayed L. Hamdi M., Biotechnol. Lett., 24/21(3), 1763–1765 (2002).
- Nishitani Y. and Osawa R., J Microbiol Methods., 54(2), 281–284 (2003).
- Graham H.N., Med., 21, 334 – 350 (1992).
- Contreras-Dominguez S. Guyot S. Marnet N. Le Petit J. Perraud-Gamie I. Roussos S. and Augur C., Biochem., 88, 1899–1908 (2006).
- Ramirez-Coronel A. Marnet N. Kumar V. Rousses S. Guyot S. and Augur C., J. Agric. Food. Chem., 52, 1344–1349 (2004).
- Libuchi S. Minoda Y. and Yamada K., Agric. Biol. Chem., 36(9), 1553–1562 (1972).
- Garcia-Conesa MT. Ostergaard P. kauppinen S. and Williamson G., Polyn ., 44(4), 319-324 (2001).
- Beverini M. and Metche M., Des. Aliments., 10, 807– 816 (1990).
- Lekha P. and Lonsane B.K., Adv. Appl. Microbial., 44, 215–260 (1997).
- Bharadwaj R. Singh B. and Bhat T.K., J. Basic. Microbiol., 43(6), 449–461 (2003).
- Sharma S. Agarwal L. and Saxena R.K., Bioresource. Technol., 99(7), 2544-2551 (2008).
- Barthomeuf C. Regerat F. and Pourrat H., J. Ferment. Technol., 77, 137–142 (1994).
- Gupta R. Bradoo S. and Saxena R.K., Appl. Microbiol., 24, 253 – 255 (1997).
- Mahapatra K. Nanda R.K. Bag S.S. Banerjee R. Pandey A. and Szakacs G., Process. Biochem., 40, 3251–3254 (2005).
- Coggon P. Graham N. and Sanderson G., Cold water soluble Tea. UK Patent 2,610,533. (1975).
- Chae S. and Yu T., Hanguk. Sipkum. Kwahakoechi., 15, 326–332 (1983).
- Pourrat H. Regerat F. Pourrat A. and Jean D., J. Ferment. Technol., 63, 401–403 (1985).
- Sharma S. and Gupta M.N., Bioorg. Med. Chem. Lett., 13(3), 395–397 (2003).
- Weetal H.H. and Detar C.C., Bioeng., 27, 124–127 (1985).
- Kar B. Banerjee R. and Bhattacharyya B.C., Process. Biochem., 37, 1395–1401 (2002).
- Yu X. Li Y. and Wu D., J. Mol. Catal. B. Enzym., 30(2), 69–73 (2004).
- Yu X.W. and Li Y.Q., J. Mol. Catal. B. Enzym., 40, 44–50 (2006).
- Kar B. Banerjee R. and Bhattacharyya B.C., J. Ind. Microbiol., 23, 173–177 (1999).
- Mukherjee G. and Banerjee R., Chim. Oggi. Chem. Today., 21(1/2), 59–62 (2003).
- Duenas M. Hernandez T. and Estrella , Food. Chem., 101, 90–97 (2007).
- Haslam E. and Stangroom J.E., J., 99(1), 28–31 (1996).

This work is licensed under a Creative Commons Attribution 4.0 International License.





