How to Cite | Publication History | PlumX Article Matrix
S. K. Umadevi*, P. Ravi, R. M. Rao Kusumanchi, Pichandy Muthuprasanna* and P. K. Lakshmi
Department of pharmaceutics, Vel’s college of pharmacy, P.V. Vaithialingam road Pallavaram, Chennai - 600 117 India.
Corresponding Author E-mail: p_ra2000@yahoo.com
ABSTRACT: Aceclofenac appears to be more active as an anti-inflammatory agent than other NSAID product and is usually well tolerated. Gels have gained more and more importance because the gel-based formulations are better percutaneously absorbed than creams and ointment bases. Therefore, aceclofenac gel formulations were made with different polymers like carbopol 934 (1 and 1.5%) and xanthan gum (4 and 5%) containing various permeation enhancers namely isopropyl myristate (IPM) (5 and 10%) and dimethyl sulfoxide (DMSO) (5 and 10%) at different proportions, having 1.5% concentration of drug. The formulated gels were evaluated for homogeneity, gelling, extrudability, pH, viscosity, drug content, spreadability, FT-IR studies and invitro diffusion studies through dialysis membrane. Invitro permeation studies were carried out for only carbopol gel formulation. Optimized gel formulation (CGF10) was tested for stability at varying temperature for two months and it was found to be more stable. A formulation containing 1.5% carbopol with 10% isopropyl myristate, showed better invitro permeation through abdominal mouse skin, and was found to be the best.
KEYWORDS: Aceclofenac; Topical gels; Carbopol; Xanthan gum; Permeation enhancers
Download this article as:| Copy the following to cite this article: Umadevi S. K, Ravi R, Kusumanchi R. M. R, Muthuprasanna P, Lakshmi P. K. The effects of polymers and permeation enhancers on in vitro release of aceclofenac from topical gel formulations. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Umadevi S. K, Ravi R, Kusumanchi R. M. R, Muthuprasanna P, Lakshmi P. K. The effects of polymers and permeation enhancers on in vitro release of aceclofenac from topical gel formulations. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6620 |
Introduction
Most of the non-steroidal anti-inflammatory drugs (NSAIDs) have been widely used for the treatment of acute and chronic arthritic conditions1. Oral doses often cause gastric irritation leading to ulcer and other systemic side effects. But the topical application of drug offers potential advantages of delivering the drug directly to site of action. The primary goal of topical product is to increase the retention of drugs in the skin rather than penetration through skin. There is growing interest in the optimization of drug delivery targeting the drug to pathological site in the skin. Creams, gels, ointments and pastes are some of the topical semisolids in use for many decades. Out of various semisolid dosage forms, the gels are becoming more popular due to ease of application and better percutaneous absorption than other semisolid preparations. Effectiveness of topical applications mainly depends upon its rate and extent of drug release from the base2.
Carbopol is a synthetic, high-molcular weight, cross linked acrylic acid polymer. Carbopol have many advantages as a gelling agent viz. high viscosity at low concentrations, stability to heat unaffected by aging, does not support microbial growth, nontoxic and nonirritant, etc 3. Carbopol was used as gel forming polymer in present study because of its suitability for topical application 4,5. Xanthan gum is a natural hydrophilic polymer, is produced by bacterial fermentation. Higher concentrations in aqueous media (1% and above) yield viscid solutions that are jelly in nature. Properties such as nontoxic, non irritant, stable and good biocompatibility make the polymer suitable for topical application.
Aceclofenac is superior from other NSAIDs as it has selectivity for cox-2, a beneficial cox inhibitor, well tolerated, better GI tolerability and improved cardiovascular safety when compared to other selective cox-2 inhibitors. It also shows increased matrix component synthesis and protection of chondrocytes against apoptosis. Aceclofenac has a faster and more potent effect than the other NSAIDs. It efficiently interferes with Neutrophils adhesion to endothelium and this effect may represent an additional relevant mechanism in its anti-inflammatory activity. Aceclofenac has an outstanding anti-inflammatory profile, involving a classical inhibition of prostaglandins E2, a decrease in the expression of several cytokines including interleukin and tumor necrosis factor. It also inhibits activated oxygen species production. In this study, Aceclofenac topical gels were prepared and the effects of polymers and permeation enhancers on invitro release of aceclofenac from topical gel formulations were investigated.
Materials and Methods
Aceclofenac (98.5-102 % pure) was gift sample from Micro labs, Banglore. Xanthan gum (viscosity of 1% aqueous dispersion 1500-2000 cps) was procured from Indian Research Products, Chennai. Carbopol 934P was purchased from SD Fine Chemicals, Chennai. Dialysis membrane-135 was obtained from Himedia Laboratories Pvt. Ltd, Mumbai. All other reagents and chemicals were of analytical grade.
Formulation of carbopol gels
As shown in table 1, a specific amount of carbopol was placed in a beaker, and sufficient amount of water was added to it and kept in oven at 100oC for 20 minutes to obtain a homogenous mixture and cooled to room temperature with continuous stirring. Methyl paraben, propyl paraben and disodium EDTA was dissolved in sufficient quantity of water and propylene glycol was added to it and this entire solution was added to the gel with continuous stirring. Drug was dissolved in alcohol and permeation enhancer (DMSO, IPM) was added to it and this solution was added to gel with continuous stirring. Finally triethanolamine was added (for neutralize purpose) drop wise with continuous stirring 6.
Formulation of xanthan gum gels
As shown in table 2, a specific amount of xanthan gum was sprinkled slowly on the surface of water little by little, whisking vigorously (If gel gets lumpy mix until smooth). When there are no lumps stop stirring. Potassium di hydrogen ortho phosphate, disodium hydrogen ortho phosphate and methyl paraben were dissolved in water with continuous stirring and propylene glycol was added to it. This solution was added to gel with continuous stirring. Drug was dissolved in alcohol and permeation enhancer (DMSO, IPM) was added to it and this solution was added to gel with continuous stirring 7.
Fourier transform infrared analysis (FT-IR)
The IR spectroscopy studies were carried out for the pure aceclofenac drug, excipients and final formulation by nujol mull method using Perkin Elmer Sprectrum one, FT-IR spectrophotometer (USA). The IR spectrum of the formulation was then analyzed with the spectrum of pure aceclofenac to assess the compatibility of the excipients with aceclofenac.
Gelling and Homogeneity
Gelling and homogeneity were tested by visual observations and assay values 8.
Extrudability
The formulations were filled in aluminum collapsible tubes. After gels are set in the container the extrudability of the formulation has been checked 9.
pH
The pH measurements were done by using a digital type pH meter by dropping the glass electrode completely into the gel system so as to cover the electrode 7. 1 gm of gel is dispersed in 100ml of water and checked for pH using pH meter.
Viscosity
The viscosity of the prepared gels was measured using Brookfield viscometer (model RVT), using spindle No. 7, at a controlled temperature of 25±2o at 70 rpm. The formulations showed torque between 50 to 90% at 70 rpm, which is the ideal range for determination of viscosity; therefore viscosity at high rpm was not determined 8.
Spreadability
The therapeutic efficacy of a formulation also depends upon its spreading value. Hence determination of spreadability is very important in evaluating gel characteristics 10. The spreadability of the gel formulations was determined 48h after preparation, by measuring the spreading diameter of 2g of the gel between two 10X10 cm glass plates after 1min. The mass of the upper plate was standardized at 50 g. The spreadability was calculated by using the formula S = m.l/t, where S is spreadability, m is the weight tied to upper slide, l is the length of the glass slide, and t is time taken.
Drug content
To ensure uniform formulation of the gel, it was sampled from the different locations in the mixer and assayed for drug content. A specified quantity of (500mg) of gel was dissolved in 50ml of methanol AR. From this solution 1ml was taken and diluted to 25ml with the same solvent. The above solution was filtered and the absorbance of solution was measured at 275nm using methanol as blank. Drug content was calculated using the equation obtained from calibration curve.
In vitro diffusion studies
In-vitro diffusion studies were performed by using Franz diffusion cell (15 ml receptor compartment capacity) using dialysis membrane. Dialysis membrane was soaked in buffer solution for 1 hour before use. Phosphate Buffer pH 6.8 was used as receptor fluid. 15 ml of phosphate buffer was taken in receptor compartment. Then 500 mg of gel containing 7.5 mg of aceclofenac was spreaded uniformly on the membrane. Dialysis membrane was mounted between the donor and receptor compartment 11. The donor compartment kept in contact with receptor compartment. The cell was then placed on to the magnetic stirrer (Kemi, India) to stir the content of receptor compartment and to maintain the temperature 37 ± 1°C. At predetermined time intervals (0.5, 1, 2, 3, 4, 6, and 8 hour), pipetted out 0.5 ml of solution from the receptor compartment and immediately replaced with the fresh 0.5 ml phosphate buffer. The above solution was further diluted to 50 ml with phosphate buffer. The samples were analyzed for the drug content at 275 nm by using phosphate buffer as blank.
In vitro permeation studies
Fresh, full thickness and hairless skin obtained from 6 to 8 weeks old mouse was used as a permeation barrier. The hair of the mice was removed 3 days before from the date of commencement of the experiment using electrical hair clipper. The animals were housed individually for at least 7 days before an experiment to allow scratches, bites other small skin imperfections to heal. After sacrificing the mice by cervical dislocation, abdominal and dorsal skin sections were excised with surgical scissors. Adhering subcutaneous fat on the dermal side was carefully removed from the under side of the skin. Skin washing was performed with phosphate buffer for 30 min 12. Then 500 mg of gel containing 7.5 mg of aceclofenac was spread uniformly on the membrane. Mouse skin membrane was mounted between the donor and receptor compartment and the experiment was continued as explained in the invitro diffusion studies.
Stability studies
The stability studies were carried out for optimized gel formula (CGF10) by keeping at different temperature conditions like ambient temperature (temperature in the working area ) 25-28°C, 2-8°C ( refrigerator temperature ), 37± 2 °C (temperature in the incubator) for 2 months. Known amount of gel were taken out at different time intervals like 0.5,1 and 2 months and was analyzed for drug content, pH and viscosity 13.
Results and Discussion
Composition and physicochemical characteristics of the gel formulations containing carbopol 934, xanthan gum as gelling agent, and different concentrations of permeation enhancers viz. IPM and DMSO, are shown in table 1 and 2. From the results it is clearly evident that all gel formulations showed good extrudability, homogeneity and spreadability. The gels formulated
Table 1: Formulae of carbopol gels.
| Ingredients | Formulation code | ||||||||||
| CGF1 | CGF2 | CGF3 | CGF4 | CGF5 | CGF6 | CGF7 | CGF8 | CGF9 | CGF10 | ||
| Aceclofenac (g) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | |
| Carbopol(g) | 1 | 1.5 | 1 | 1 | 1.5 | 1.5 | 1 | 1 | 1.5 | 1.5 | |
| Alcohol(ml) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | |
| Propylene glycol(ml) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| DMSO(ml) | – | – | 5 | 10 | 5 | 10 | – | – | – | – | |
| Isopropyl myristate(ml) | – | – | – | – | – | – | 5 | 10 | 5 | 10 | |
| Methyl paraben(mg) | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | |
| Propyl paraben (mg) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | |
| Disodium EDTA(mg) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | |
| Triethanolamine(ml) | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | |
| Purified water(ml) | q.s. | q.s. | q.s | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | |
| Physicochemical properties | |||||||||||
| Homogeneity | ++
|
+++
|
++
|
++
|
++
|
+++
|
++
|
++
|
++
|
+++
|
|
| Gelling | ++
|
+++
|
++
|
++
|
++
|
+++
|
++
|
++
|
++
|
+++
|
|
| Extrudability | ++
|
+++
|
++
|
++
|
++
|
+++
|
++
|
++
|
++
|
+++
|
|
| pH | 6.99 | 7.14 | 6.90 | 6.99 | 7.09 | 7.18 | 6.94 | 6.97 | 7.02 | 6.89 | |
| Viscosity in cps | 8650 | 9150 | 8420 | 8350 | 8800 | 8650 | 8450 | 8250 | 8750 | 8500 | |
| Spreadability(g.cm/sec) | 38.46 | 50.00 | 35.71 | 41.66 | 38.46 | 45.45 | 35.71 | 38.46 | 45.45 | 50.00
|
|
| Drug content | 97.50 | 97.70 | 99.50 | 99.10 | 100.23 | 97.50 | 97.50 | 98.70 | 99.10 | 99.50 | |
The values indicate mean + SD; N= 6; +++ excellent; ++ good.
with xanthan gum showed pH values between 6.4 to 6.8, viscosity range from 14,100±36 to 16,600±72 cps, and their drug content was in the range of 93.08 ±0.12 to 101.42±0.34. The gels formulated with carbopol showed pH values between 6.92 to 7.2, viscosity range from 8,250±70 to 9,150±61 cps , and their drug content was in the range of 97.50±0.4 to 100.23±0.26. pH of the all gel formulations lies in the normal pH range of skin. The viscosity of all gel formulations was found to decrease with increase in permeation enhancer concentration. The drug content determination also showed that the drug was uniformly distributed throughout gel.
Table 2. Formulae of xanthan gum gels
| Ingredients | Formulation code | |||||||||
| XGF1 | XGF2 | XGF3 | XGF4 | XGF5 | XGF6 | XGF7 | XGF8 | XGF9 | XGF10 | |
| Aceclofenac (g) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Xanthan gum(g) | 4 | 5 | 4 | 4 | 5 | 5 | 4 | 4 | 5 | 5 |
| Alcohol(ml) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Propylene glycol(ml) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| DMSO(ml) | – | – | 5 | 10 | 5 | 10 | – | – | – | – |
| Isopropyl myristate(ml) | – | – | – | – | – | – | 5 | 10 | 5 | 10 |
| Methyl paraben(mg) | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
| Potassium dihydrogen
Ortho phosphate (g) |
0.908 | 0.908 | 0.908 | 0.908 | 0.908 | 0.908 | 0.908 | 0.908 | 0.908 | 0.908 |
| Disodium hydrogen
Ortho phosphate(g) |
2.38 | 2.38 | 2.38 | 2.38 | 2.38 | 2.38 | 2.38 | 2.38 | 2.38 | 2.38 |
| Purified water(ml) | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Physicochemical properties | ||||||||||
| Homogeneity | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +++ |
| Gelling | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +++ |
| Extrudability | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +++ |
| pH | 6.62 | 6.80 | 6.53 | 6.80 | 6.72 | 6.80 | 6.41 | 6.62 | 6.70 | 6.80 |
| Viscosity in cps | 15450 | 16600 | 14820 | 14450 | 16100 | 15610 | 14950 | 14100 | 16200 | 15750 |
| Spreadability | 50.00 | 55.55 | 45.45 | 38.46 | 41.66 | 50.00 | 50.00 | 55.55 | 45.45 | 50.00 |
| Drug content | 93.08 | 97.97 | 99.19 | 96.45 | 101.42 | 99.11 | 98.05 | 94.15 | 96.12 | 97.92 |
The values indicate mean + SD; N= 6; +++ excellent; ++ good.
The comparative analysis of FT-IR spectrum of the standard drug, physical mixtures of excipients with drug and the formulations revealed that there was no incompatibility between the drug and excipients.
Figure 1 and 2 reflects the invitro diffusion profile of aceclofenac from gels containing different concentrations of xanthan gum, and permeation enhancers DMSO and IPM. On comparing carbopol gels with xanthan gum gels, it is obvious that xanthan gum gels were less efficient in releasing the drug, for example 1.5% carbopol gel (CGF10) released maximum of 93.01±0.50% of aceclofenac over a period of 8 hour whereas 5% xanthan gum gel released only 64.72±0.76%. So, for invitro permeation studies gels with carbopol were selected.
 |
Figure 1: Diffusion profile of xanthan gum from various formulations with and without permeation enhancers DMSO.
|
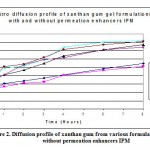 |
Figure 2. Diffusion profile of xanthan gum from various formulations with and without permeation enhancers IPM.
|
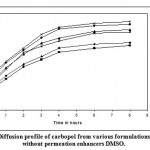 |
Figure 3: Diffusion profile of carbopol from various formulations with and without permeation enhancers DMSO.
|
Figure 3 and 4 depicts the invitro diffusion profile of aceclofenac from gels containing different concentrations of carbopol 934P, and permeation enhancers DMSO and IPM. The total amount of drug release for a fixed period of 8hrs was found to decrease with increase in carbopol concentration. Even though a good drug release was observed with 1% carbopol, as it was soft and less viscous in nature, and an optimum polymer concentration of 1.5%, which showed good consistency, was found to be suitable. On comparing the diffusion profile of carbopol gel containing different permeation enhancer viz. IPM and DMSO in different concentrations, it indicates that the IPM containing formulations showed better diffusion of drug through dialysis membrane, compared to DMSO containing gel formulations.
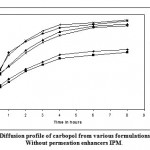 |
Figure 4: Diffusion profile of carbopol from various formulations with and Without permeation enhancers IPM.
|
Figure 5 and 6 depicts the invitro skin permeation profile of aceclofenac from carbopol gels. The skin permeation showed a similar pattern as that of the diffusion profile; but the % cumulative amount of drug release through dialysis membrane was more when compared with permeation studies through mouse skin. In diffusion studies as well as permeation studies, after 0.5 hour burst release of drug was observed in all gel formulations, Because of presence of higher concentration of ethanol and permeation enhancer in the donor compartment. After 6th hour drug release was slow, which may be due to the depletion of ethanol and permeation enhancer.
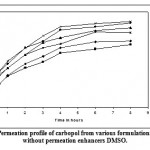 |
Figure 5: Permeation profile of carbopol from various formulations with and without permeation enhancers DMSO.
|
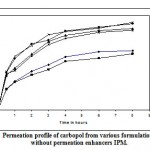 |
Figure 6 Permeation profile of carbopol from various formulations with and without permeation enhancers IPM.
|
From the permeation result, the gel formulation prepared with 1.5% carbopol 934P containing 10% IPM as enhancer (CGF10), was found to be most satisfactory, compared to other formulations respect to physicochemical properties. So, formulation CGF10 was subjected to stability studies for two months. The results of stability studies are in table 4. There were no significant changes were observed in the characteristics (drug content, viscosity and pH) of CGF10 formulation after storing at different temperature conditions for two months.
Table 4. Stability studies of optimized (CGF10) formulation.
| Temperature(°C) | Time (months) | Drug content (%) | Viscosity in cps | pH |
| 2 – 8°C | 0 | 99.50±0.06 | 8500±20 | 6.98±0.08 |
| 0.5 | 100.50±0.21 | 8481±39 | 6.92±0.23 | |
| 1 | 99.40±0.13 | 8568±67 | 6.96±0.17 | |
| 2 | 99.80±0.18 | 8524±44 | 6.99±0.40 | |
| 25 – 28°C | 0 | 99.50±0.06 | 8500±20 | 6.98±0.08 |
| 0.5 | 99.43±0.32 | 8612±45 | 6.91±0.34 | |
| 1 | 99.62±0.14 | 8641±21 | 6.84±0.43 | |
| 2 | 99.56±0.28 | 8584±65 | 6.94±0.19 | |
| 37± 2 °C | 0 | 99.50±0.06 | 8500±20 | 6.98±0.08 |
| 0.5 | 99.19±0.11 | 8650±67 | 6.90±0.22 | |
| 1 | 99.56±0.49 | 8691±71 | 6.86±0.76 | |
| 2 | 99.58±0.15 | 8656±81 | 6.89±0.05 |
Drug content, Viscosity and pH values are expressed as Mean±S.D; n=3.
Conclusions
In vitro studies of the xanthan gum gel formulations showed lesser rate of release than that of carbopol gel formulations. From the above results, it can be concluded that the gel formulation (CGF10) prepared with 1.5% carbopol 934 containing 10% IPM as enhancer was found to be the most satisfactory, compared to other formulations with respect to good drug release and physicochemical characteristics. Thus, IPM can be a better permeation enhancer for aceclofenac than DMSO in topical gels. Hence further studies such as preclinical and clinical trails will certainly be beneficial in the development of optimized formulation (CGF10) in the treatment of rheumatoid arthritis more effectively.
Acknowledgement
The authors are thankful to Dr. Ishari K. Ganesh, the chairman, Vel’s college of pharmacy, Chennai, for providing facilities for the research work, micro labs, Banglore for providing the providing gift sample of drug.
References
- Forsgreen, J.NSAID in rheumatoid arthritis, Scand J.Rheumatol., 14:7 (1976)..
- V.Loganathan, S.Manimaran, A.Jaswanth, The effects of polymers and penetration enhancers on release of flurbiprofen from gel formulations. Indian Journal of Pharmaceutical Sciences., 63(3):200-204 (2001). .
- Nairn, J.G, Encyclopedia of Pharmaceutical Technology, Marcel Dekker, lnc., New York, 15:224 (1997). .
- D.W.Osborne, Pena L.E, Gel dosage forms: Theory, Formulation and processing In Topical drug delivery formulations, Marcel Dekker, New York, 381-388 (1990).
- Klech, C.M, Gels and jellies: Encyclopedia of Pharmaceutical Technology, Marcel Dekker., lnc., New York, 415-439 (1992).
- G.D Gupta, R.S gaud, Anti-inflammatory activity of tenoxicam gel on carrageenan-induced paw oedema in rats, Indian Journal of Pharmaceutical Sciences., 68(3):356-359 (2006).
- Mundada A.S, Shri kande B.K, Controlled release of ciprofloxacin HCl for ophthalmic administration, Indian Journal of Pharmaceutical Sciences., 43(1):9-12 (2005).
- L.Panigrahi, S.K.Ghosal, Snigda pattnaik, Effect of permeation enhancers on the release and pharmaco kinetics of lincomycin hydrochloride gel formulations through mouse skin, Indian Journal of Pharmaceutical Sciences., 68(2):205-211 (2006).
- Libermann H.A.,rieger, Banker G.S, Pharmaceutical Dosage Forms: Disperse systems. Mercel-Dekker, Newyork, 594 (1989).
- Barry B.W, Meyor, M.C, Sensory assessment of spreadability of hydrophilic topical preparations, Journal of Pharmaceutical Sciences., 62(8):1349-1354 (1973).
- Nappinnai M, pakalapati.S, Armilli R, Rofecoxib gels-preparation and evaluation, Indian Drugs., 43(6):513-515 (2005).
- M.Sreenivasa Reddy, S.Mutalik, G.Veerabhadra Rao, Preparation and evaluation of minoxidil gels for topical application in alopecia, Indian Journal of Pharmaceutical Sciences., 68(4):432-436 (2006).
- S.H.Nayak, P.D. Nakat, P.G.yeole, Development and evaluation of cosmeceutical hair styling gels of ketaconazole, Indian Journal of Pharmaceutical Sciences., 231-233 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.





