How to Cite | Publication History | PlumX Article Matrix
E.M.R. Kiremire1*, N. Hamata1, K. Chibale2 and H. Kambafwile2
1Department of Chemistry, University of Namibia, Private Bag 133 01 Namibia.
2Department of Chemistry, University of Cape Town, Rondebosch 7701 South Africa.
ABSTRACT: Transition metal complexes derived from biologically active ligands and selected metal ions have been synthesized. They were characterized by microanalysis, Fourier Transform infrared (FT-IR), proton NMR (1HNMR) and Low Resolution Mass Spectrometry (LRMS) techniques. The paramagnetic shift influence observed in some of the complexes is qualitatively discussed.
KEYWORDS: Transition metal complexes; Microanalysis; FTIR; HNMR; LRMS
Download this article as:| Copy the following to cite this article: Kiremire E. M. R, Hamata N, Chibale K, Kambafwile H. The synthesis and characterization of new metal complexes from biologically active thiosemicarbazone ligands bound to metal ions. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Kiremire E. M. R, Hamata N, Chibale K, Kambafwile H. The synthesis and characterization of new metal complexes from biologically active thiosemicarbazone ligands bound to metal ions. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6441 |
Introduction
Intense research involving thiosemicarbazones is currently going on in many laboratories worldwide due to the wide range of biological activities exhibited by the compounds1-10. A substantial number of thiosemicarbazones have been found to be biologically active against protozoa, bacteria, viruses and fungi. The list of infections studied, among others, include tuberclosis, leprosy, psoriasis, rheumatism, trypanosomiasis, cossidiosis, leukemia, cancer, Chagas disease, leishmaniasis, Herpes Simplex virus, Neisseria gonorrhea and Neissera meningitidis1-11. Furhermore, structural activitity relationship (SAR) studies have been conducted on a selected range of thiosemicarbazones1,9. Our recent studies indicated that a dithio thiosemicarbazone when complexed to certain metal ions produced complexes with high biological activity10. In our continued investigations, it was decided to focus our attention in synthesizing the metal complexes utilizing the ligands shown in figure 1 with a view to carrying out biological tests against malarial parasite.
We hereby report the synthesis and characterization of new metal complexes of nickel(II), copper(II), and zinc(II) containing 2-acetylpyridine-4-phenyl-3-thiosemicarbazone and 2-acetylpyridine and 2-formylpyridine thiosemicarbazone
Experimental
All the chemicals used were purchased from Aldrich and used as received.
The spectroscopic measurements FT-IR, 1 HNMR , Microanalyses, and Low Resolution Mass Spectroscopy were done at the University of Cape Town.
Preparation of the Ligands
The synthesis of 2–Acetylpylridine-4–Phenyl–3– thiosemicarbazone (LH)
The thiosemicarbazone ligands were prepared according to a literature method12 which involved a condensation reaction.The thiosemicarbazone ligands where prepared through a condensation reaction.The reactants were taken in the mole ratio of 1:1. Thus, 2–Acetylpylridine-4–Phenyl–3– thiosemicarbazone(LH) was prepared by adding 2-acetylpyridine (2.0 ml) to 4-phenyl 3-thiosemicarbazide (2.982g) in methanol (125ml) followed by acetic acid (equivalent of 1% of the total volume of the mixture) and then refluxing for 3 hours. The reaction mixture was cooled and the thiosemicarbazone precipitated out. It was then filtered off, dried on the pump for 60 minutes and then recrystallized from ethanol.
The yield obtained was 1.10 g (36%).
The synthesis of 2-Pyridine Carboxaldehyde -4– Phenyl –3– thiosemicarbazone
( 2-formylpyridine-4-phenyl-3-thiosemicarbazone, GH)
The formythiosemicarbazone ligand (GH) was prepared by dissolving of 4-phenyl 3-thiosemicarbazide (3.516g) in methanol (150ml) and adding 2-Pyridine carboxaldehyde (2.0 ml) then refluxing the mixture for 20 minutes after adding acetic acid as in the previous case. Upon cooling the precipitate formed was filtered off, dried at the water pump for 45minutes. The ligand was not recrystallised due to it insolubility in most solvents. The yield was 4.25 g(79%).
Preparation of Thiosemicarbazone Complexes
Preparation of the Copper complex, CuLCl
CuCl2.2H2O (0.3g) was dissolved in of distilled water (25 ml) while the the ligand (0.414g) was dissolved in ethanol (130 ml).The two solutions were mixed by adding slowly the cupric solution to the ligand solution with continuous stirring.
A green precipitate which formed was allowed to settle. It was then filtered off, washed with water, ethanol and ether and then allowed to dry at the pump for 30 minutes. The complex was recrystallized from acetone. The yield was 0.56 g(86%).
Preparation of the Zinc complex, ZnL(LH)Ac
(CH3-COO)2 Zn.2H2O (0.3g) were dissolved in distilled water (20 ml) and the ligand (0.423g) was dissolved in ethanol (200 ml). Upon mixing the two solutions a yellow precipitate formed. It was then filtered off, washed with water, ethanol and ether, and then allowed to dry at the water pump for 30 minutes. The complex formed was not recrystallised due to the lack of a suitable solvent.
The yield was 0.22 g(24%).
Preparation of the Nickel complex, NiL2
Two solutions prepared by dissolving NiCl2. 6H2O (0.3g) and the ligand (0.681g) in distilled water (15 ml) and in ethanol (140 ml) respectively were mixed. Upon adding the metal solution to the ligand solution a fine brown precipitate was produced. A little excess of NiCl2. 6H2O was added to complete the precipitation.
The precipitate was filtered off, washed with water, ethanol and ether. It was then air-dried at the water pump for 30 minutes. The complex was also found to be insoluble in the common solvents tried. The yield obtained was 0.70 g(93%).
Preparation of 2-formylpyridine-4-phenyl-3-thiosemicarbazone,
(GH) Complexes
Preparation of the Copper complex, CuGCl
Two solutions were prepared by dissolving of CuCl2.2H2O (0.3g) in distilled water (25ml) and the ligand (0.451g) in Dimethyl Sulphoxide (DMSO) (70 ml). The metal solution was then attached to the ligand solution with stirring. A dark green precipitate was immediately formed. It was then filtered off, washed with water, ethanol and then ether and allowed to dry at the water pump for 45 minutes. The complex was also found to be insoluble in the common solvents used.
It yield was 0.30 g(48%).
The preparation of the Zinc complex, ZnG(GH)Ac
Two solutions were prepared by dissolving (CH3-COO)2 Zn.2H2O (0.30 g) in distilled water (30 ml), while the ligand (0.351g) was dissolved in DMSO (60ml). The metal solution was slowly added to the ligand solution with stirring.The bright yellow precipitate which was formed was filtered off, washed with water, ethanol and ether. It was then dried at the water pump for 45 minutes. The complex was also found to be insoluble in the common solvents. The yield obtained is 0.11 g(13%).
Results and Discussion
The metal complexes of nickel(II), copper(II), and zinc(II) containing 2-acetylpyridine-4-phenyl-3-thiosemicarbazone and 2-acetylpyridine and 2-formylpyridine thiosemicarbazone were characterized by microanalysis, Low Resolution Mass Spectrometry(LRMS), proton NMR and Fourier Transform IR techniques. The results are presented in Tables 1-6 and Figures 4-8. These results are consistent with the formation of the ligands LH and GH as well as NiL2, ZnL(LH)Cl, CuLCl, ZnG(GH)Cl, and CuGCl complexes.
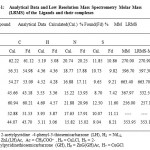 |
Table 1: Analytical Data and Low Resolution Mass Spectrometry Molar Mass (LRMS) of the Ligands and their complexes.
|
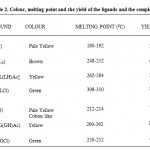 |
Table 2: Colour, melting point and the yield of the ligands and the complexes.
|
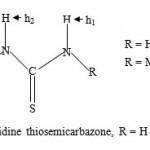 |
Figure-1: 2-Acetylpyridine thiosemicarbazone, R = H or Me.
|
Paramagnetic Shift Influence
We recently discerned paramagnetic shift influence in the ferrocene-based thiosemicarbarzone copper(II) complexes13. The 1HNMR spectral data of the ligand LH (H1) and the zinc complex ZnL(LH)Ac (H3) are given in Table 2. For LH(CDCl3 solvent), the signals at values 2.446 and 2.477 ppm can tentatively be assigned to the methyl fragments CH3. The signal at value of 2.446 could be attributed to CH3 of the ligand fragments and the signal at value of 2.477 could be attributed to the Me fragment from the acetate ion, CH3COO− and S-H tautomer (see figure 2). The clustered NMR signals centered around 7.20-7.5.0 and 7.60-8.00 ppm ranges may be assigned to the phenyl and pyridine hydrogens respectively. The pyridine hydrogens NMR peaks have been observed to appear at higher values in the NMR spectra14. The broad triplet signal centered at 8.60-8.90 value range may be attributed to the h1 hydrogen. The splitting is likely due to the coupling with 14N( I = 1, MI = 1,0,-1) nitrogen. The doublet signal at values of 9.40 and 9.60 ppm may be assigned to h2 hydrogen. Unlike the h1 hydrogen, the signal has not been resolved into triplets. The proton NMR spectrum of ZnL(LH)Ac using DMSO as a solvent is also shown in Table 2. The signal at 2.503 ppm can be assigned to DMSO. The peak at 2.708 ppm is assigned to Me fragment of the ligands. The signal at 3.280 may be assigned to the Me fragment of the CH3COO− and S-H hydrogen arising from the tautomerism of the ligand in solution. A similar signal which may be assigned to S-H tautomerism(see figure 2) is also found in the proton NMR of 2-formylpyridine thiosemicarbazone (DMSO solvent) which has no methyl fragment. The multiplet peaks in the range 6.90-7.40 ppm may be assigned to the Ph fragment while the one with an absorption in the value range of 7.80-8.00 can be assigned to the pyridine ring (Py). The last peak at value of 9.220 ppm may be assigned to the h1 hydrogen. The Zn(II) ion has a d10 configuration, and hence there are no unpaired electrons. In this case the complex will not show paramagnetic shift influence.
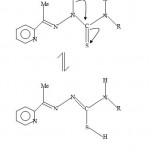 |
Figure-2: 2-Acetylpyridine thiosemicarbazone tautomerism.
|
The proton NMR spectrum of CuLCl (H4 ) complex in CDCl3 showed one broad large signal at value of 1.56 ppm and a smaller peak at 1.30 ppm. These could be due to the Me fragments of the ligand stereoisomers arising from the presence C=N fragment in the molecule. The signals due to Ph and Py fragments were very much obscured due to paramagnetic shift influence of Cu(II) ion (d9) which has one unpaired electron . However, when D2O was used as a solvent, weak broadened peaks were observed at values of 1.806, 2.433, 4.647(D2O), 7.60, 7.90,8.10 and 8.50( see Table 4). The first two peaks may be assigned to the Me stereoisomers, while the last four may be assigned to Ph and Py fragments. The N-H (h1) hydrogen signal was completely submerged. Thus, it appears that paramagnetic shift influence is also influenced by the nature of the solvent used.Finally, by comparing the 1HNMR of H1(LH) and H3[ZnL(LH)Ac] Figures 4 and 5 respectively, it is quite clear that the peaks in the zinc complex have a better resolution possibly due to ligand coordination despite the absence of paramagnetic shift influence in the complex.
Table 3: 1HNMR of H1 (CDCl3 )and H3 (DMSO) , ( -values, ppm).
| H1 (CDCl3) | Tentative Assignment | H3 (DMSO) | Tentative Assignment |
| 2.446 | Me | 2.503 | DMSO |
| 2.477
7.20-7.50 (m) |
Me | 2.708 | Me |
| [7.381,7.385,7.409] Ph | Py | 3.280 | S-H/Ac |
| 7.60-8.00 (m) | Py | 7.80-8.00 (m) Ph | |
| 8.60-8.90 (t ) | h1 | 9.220 | h1 |
| 9.40-960 (d) | h2 |
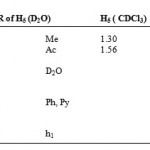 |
Table 4: 1HNMR of H5 (D2O) H5 ( CDCl3).
|
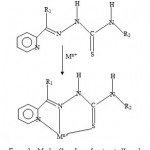 |
Figure 3a: Mode of bonding of protonated ligand.
|
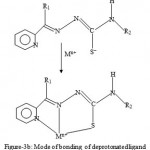 |
Figure 3b: Mode of bonding of deprotonated ligand.
|
Infrared Spectra
The FT-IR spectra of H1(LH) ,H2(NiL2), H3[ZnL(LH)Ac], H6[ZnG(GH)Ac], and H7(CuGCl) are given in Tables 4 and 5 and representative spectra shown in Figures 6-8. The spectra show peaks mainly characteristic of the functional groups C-H( alkyl), C-H( aromatic), C=N, C=S, C-N and NH functional groups. The peaks for LH(H1) can tentatively be assigned14-15 as follows,
 |
Figure 4: Proton NMR of H1(LH).
|
![Figure 5: Proton NMR of H3 [ZnL(LH)Ac].](https://www.biotech-asia.org/wp-content/uploads/2016/02/Vol_5-no1_the_kir_fig5-150x150.jpg) |
Figure 5: Proton NMR of H3 [ZnL(LH)Ac].
|
 |
Figure 6: IR spectrum of H1(LH).
|
N-H( 3367, 3333 cm-1), C-H(3064, 3019, 2986, 2850), C=N( 1698), benzene and pyridine rings (1596-1419), C-H(1466, 1446), and C-H bends(1179-1107). In NiL2 (H2 ) complex, the loss of one of the N-H hydrogens gives rise to the decreased intensity and a small weak peak at 3415 cm-1. In the case of the zinc complex [ZnL(LH)Ac](H3), both L− and LH have N-H hydrogens which exhibit weak peaks at 3417 and 3321 cm-1. The zinc(II) complex , [ZnG(GH)Ac](H6) has a medium peak at 3416 cm-1 due to N-H. Finally, the copper(II), CuGCl(H7) complex shows a weak peak around 3400cm-1.due to the N-H fragment.
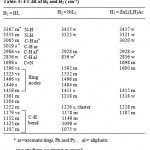 |
Table 5: FT-IR of H1 and H2 ( cm-1).
|
Bonding and Possible Molecular Geometries of the Complexes
Nearly all the 2-acetylpyridine thiosemicarbarzones and 2-formylpyridine thiosemicarbarzone complexes known so far and whose structures have been determined by x-ray analysis, the ligands have been found to exhibit a tridentate mode of coordination. This is shown in figures 3a and 3b.The geometry of NiL2, is probably a distorted octahedral as was recently found for the cadmium complex16, CdL0 2. As already stated, the deprotonated ligand L− or G− normally bonds to a metal in a tridentate fashion17-18. In the case of ZnL(LH)Ac complex, both the L− and LH ligand may act as a tidentate while Ac− acting as a counter ion, or the complex may comprise of L− acting as a tridentate and LH as a bidentate and Ac− as a monodentate. The same argument applies for the possible geometry of ZnG(GH)Ac. In the case of the CuLCl or CuGCl complex, the plausible geometry adopted is probably a distorted tetrahedral one with L− or G− acting as a tridentate ligand and the fourth coordination site being occupied by Cl−. Further work is in progress to elucidate the geometries of the complexes by x-ray analysis and to determine whether or not the complexes possess biological activity.
| H6 = ZnG(GH)Ac | H7 = CuGCl |
| 3416 w | 3400 w |
| 3007 s | 3013 w |
| 2928 vs | 2928 m |
| 2855 s | 2855 w |
| 2100 w, br | |
| 1697 m | |
| 1620 m | 1681 w |
| 1601 s | 1594 m |
| 1525 m | 1542 m |
| 1498 s | 1492 w |
| 1448 m | 1456 w |
| 1404 vs | 1425 m |
| 1354 m | |
| 1313 m | |
| 1292 w | |
| 1219 m, cluster | 1226 vs, cluster |
| 1181 m | |
| 1153 w | |
| 1131 m | 1131 w |
| 1100 w | |
| 1050 m |
![Figure 7: IR spectrum of H6 [ZnG(GH)Ac] complex.](https://www.biotech-asia.org/wp-content/uploads/2016/02/Vol_5-no1_the_kir_fig7-150x150.jpg) |
Figure 7: IR spectrum of H6 [ZnG(GH)Ac] complex.
|
 |
Figure : IR spectrum of H2(NiL2) complex.
|
Conclusion
New metal complexes containing formylpyridine-4-phenyl-3-thiosemicarbazone(GH) and 2-acetylpyridine-4-phenyl-3-thiosemicarbazone(LH) ligands have been synthesized.
The complexes are CuLCl, ZnL(HL)Ac, NiL2, CuGCl and ZnG(GH)Ac. They were characterized by elemental analysis, FT-IR, Low Resolution Mass Spectrometry and 1HNMR.
Acknowledgement
We wish to thank the University of Namibia and Petrofund Namibia for funding. The extend our sincere thanks to the University of Cape Town for the continued provision of facilities for spectroscopic measurements.
References
- Klayman, D. L., Bartosevich, J. P., Griffin, T.S., Mason, C. J., Scovill, J. P., J. Med. Chem.,22(7), 855(1979).
- Klayman, D. L.; Scovill, J. P.; Bartosevich,Mason, C. J. J. Med. Chem. 22(11): 1367 (1979).
- Casero, Jr.,R. A., Klayman, D. L., Childs, G.E., Scovill, J. P., Desjardins, R. E., Antimicrobiol Agents and Chemotherapy, 18(2): 317 (1980).
- Lambros, G. E., Childs, G. E., Notsch, J. D.,Scovill, J. P., Klayman, D. L., Davidson, Jr.,D. E., Antimicrobiol Agents & Chemotherapy, 22(6): 981 (1982).
- Klayman, D. L., Scovill, J. P., Bartosevich, J.F., Bruce, J., J. Med. Chem., 26: 35 (1983).
- Scovill, J. P., Klayman, D. L., Lambros, C.,Childs, G. E., Notsch, J. D., J. Med. Chem.,27: 87(1984).
- Gómez-Bosquet, M.; Moremo, V.; Font-Bardia, M.; Solans, X.; Metal-based drugs, 5(3): 161 (1998).
- Du, X., Guo, C., Hansell, E., Doyle, P. S.,Caffrey, C. R., Holler, T. P., McKerrow, J. H.,Cohen, F. E., J. Med. Chem., 45: 2695 (2002).
- Greenbaum, D. C., Mackey, Z., Hansell, E.,Doyle, P., Gut, J.; Caffrey, J. L., Rosenthal,P. J., McKerrow, J. H., Chibale, K., J. Med.Chem., 47: 3212 (2004).
- Kiremire, E. M. R., Chibale, K. , Rosenthal, P. J., Daniel, L. S. Negonga, A. M. and Munyolo, F. M., Biosciences, Biotechnology Research Asia Vol. 4(2): 401 (2007).
- Brown, R. E., Stancato, F.A., and Wolfe, A. D., Antimicrobiol Agents and Chemotherapy, 19(2): 234 (1981).
- Graúdo, J. E. J.C., Filgueiras, C. A. L., Marques-Netto, A., and Batista, A. A., J. Braz. Chem. Soc. 11(3): 237(2000).
- Kiremire, E. M. R., Daniel, L. S., Mu Ashekele H., Chibale, K., Kambafwile, H., Oriental Journal of Chemistry, 23(3): 785(2007).
- Williams, D. H., and Fleming, I., “Spectroscopic Methods in Organic Chemistry”, 5th Edition, The McGraw-Hill Companies, New York, (1995).
- Smith,B, “Infrared Spectral Interpretation”, CRC Press, London (1999).
- Kiremire, E. M. R., Chibale, K., Likius, D. S., Su, H. and Kambafwile, H., Oriental Journal of Chemistry, 23(2): 415(2007).
- Gómez-Saiz, P., Garcίa-Tojal, J., Mendia, A., Donnadiu, B., Lezama, L., Pizzaro, J., L., Arriortua, M., I. and Rojo, T., Eur. J. Chem., 518(2003).
- Kang, Y., Kang, S. O., Ko, J. and Sohn, J., Bull. Korean Chem. Soc., 20(1) : 65(1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.





