How to Cite | Publication History | PlumX Article Matrix
S. Suman1* and K. Ramesh2
1Department of Pharmaceutical Biotechnology, Karnataka College of Pharmacy, Bangalore India.
2Department of Pharmaceutics, Karnataka College of Pharmacy, Bangalore India.
Corresponding Author E-mail: suman412b@rediffmail.com
ABSTRACT: Isolation and identification of thermophilic Bacillus sp was carried out from soil samples of a tannery area. The isolate KCPSS-3, when cultivated in liquid cultures containing trisodium citrate, produced protease. The production reached a maximum in 9 h, with levels of 2.15 U/mg protein. The microorganism utilized several carbon sources for the production of protease. Starch was found to be the best substrate, followed by trisodium citrate, citric acid and sucrose. Among the various organic and inorganic nitrogen sources used, ammonium nitrate was found to be the best. Studies on the effects of temperature and pH on protease production revealed that the Bacillus sp could grow up to 65°C within a broad pH range of 5-10 with an optimal growth temperature and pH at 60°C and 8.0, respectively. Thermostability studies revealed that the enzyme was stable for 2 h at 30°C, while at 40°C and 80°C, 14% and 84% of the original activities were lost. Hence, it’s an indication that the enzyme produced is thermostable.
KEYWORDS: Protease; thermophilic bacterium; Bacillus sp.
Download this article as:| Copy the following to cite this article: Suman S, Ramesh K. An Extracellular Thermostable Protease Production from Thermophilic Bacillus Species Isolated from Soil. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Suman S, Ramesh K. An Extracellular Thermostable Protease Production from Thermophilic Bacillus Species Isolated from Soil. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7500 |
Introduction
Proteases are one of the three largest groups of industrial enzymes. They account for nearly 60 % of the total enzyme sales covering about 20 % of the world market, and they are used mainly in detergents (3, 8, 10). Among the various proteases, bacterial proteases are the most significant, compared with animal and fungal proteases (9) and among bacteria, Bacillus sp are specific producers of extra-cellular proteases (6). These enzymes have wide industrial application, including pharmaceutical industry, leather industry, manufacture of protein hydrolyzates, food industry and waste processing industry (4).
Global requirements of thermostable biocatalysts are far greater than those of the mesophiles of which proteases contribute two thirds (8). Thermostable proteases are advantageous in some applications because higher processing temperatures can be employed, resulting in faster reaction rates, increase in solubility of nongaseous reactants and products, and reduced incidence of microbial contamination by mesophilic organisms (2).
In this study, bacteria isolated from soil samples collected from different places from a tannery area, was screened for proteolytic activity. The selection of medium components for the optimal production of extracellular protease by thermophilic Bacillus sp strain KCPSS-3, along with some biochemical properties of the enzyme is also described. The effects of pH on protease production and temperature on the activity and stability of the enzyme are also carried out.
Materials and Methods
Organism
The bacterial strain used in this study was the thermophilic Bacillus sp strain KCPSS-3, previously isolated from soil samples collected from different places from a tannery area. Growth in 5% nutrient broth at pH 5-8 and temperature 37-70 0C were examined. Different biochemical tests were employed for its identification.
Screening for proteolytic activity
The bacterial isolates were plated onto nutrient broth-casein-agar medium and were incubated at 37 0C for 24 h. The strain was screened on the basis of formation of a clear zone of casein hydrolysis on the test plate and was transferred to nutrient agar slant for growth and maintenance.
Protease production
The culture medium used in this investigation for protease production contained: 0.5% w/v D-glucose, 0.75% w/v peptone, 0.5% w/v MgSO4.7H2O, 0.5% w/v KH2PO4, and 0.01% w/v FeSO4.7H2O maintained at 37 0C for 24-72 h in a shaker incubator (120 rpm).At the end of each fermentation period, the whole fermentation broth was centrifuged at 10,000 rpm at 4 0C for 15-20 min and the clear supernatant was used as crude enzyme preparation.
Effect of culture conditions on enzyme production
The effects of carbon sources 1% w/v on enzyme production were investigated replacing trisodium citrate by glycerol, lactose, D(+)galactose, sucrose, maltose, starch, D(+)glucose, casein and citric acid. Different nitrogen sources including NH4NO3, peptone, yeast extract, casein, (NH4)2SO4, NH4Cl, KNO3 and ammonium citrate were employed for preliminary studies to determine growth and production of extracellular protease.
Determination of protease activity
The activity of protease was assessed in triplicate by measuring the release of trichloroacetic acid soluble peptides from 0.5% w/v azocasein in Tris-HCl buffer (pH 7.2) when 500 μl of the same was incubated with 100 μl of the enzyme for 60 min at 37 0C. The reaction was stopped by adding 500 μl of 15% TCA with shaking. This was left for 15 min and then centrifuged at 5000 rpm at 4 0C for 15-20 min. 1 ml of the supernatant was added to 1 ml of 1M NaOH and the absorbance was read at 440 nm. One unit (U) of protease activity was defined as the number of micromole of substrate converted to product per minute under standard assay conditions.
Effect of pH on protease production
The effect of pH on protease production from Bacillus sp was determined by growing each species in fermentation medium of different pH using appropriate buffers: phosphate buffer (pH 5-6.0), Tris-HCl buffer (pH 7.0-8.0) and glycine-NaOH buffer (pH 9-10). Protease production was measured and monitored every 6 h over a period of 72 h of fermentation through assay of protease activity.
Effect of temperature on protease production
The effect of temperature on protease production was studied by growing each Bacillus sp in fermentation media set at different temperatures (37, 45, 50, 55, 60, 65 and 70 0C). Protease production was monitored every 6 h over a period of 72 h of fermentation through protease activity assay.
Effect of temperature on activity and stability of enzyme
The effect of temperature on the enzyme activity was determined by performing the standard assay procedure at pH 7.5 at different temperatures (37, 45, 50, 55, 60, 65 and 70 0C). Thermostability was determined by incubating the crude enzyme at above mentioned temperatures for 2 h in a thermostatic water bath. After treatment the residual enzyme activities were assayed.
Results and Discussion
Effect of culture conditions on enzyme production
The growth pattern of Bacillus sp. KCPSS-3 and protease production was observed for 12 h in liquid medium with 1% trisodium citrate as a carbon source in a 250 ml conical flask (Fig. 1). Bacillus sp grew very fast and the formation of protease started from 5th hour of the growth and reached a maximum in 9 h (2.15 U/mg protein) then began to fall. This implies that the production of protease was directly linked to the metabolically active state of the culture. Ward (9) reported that Bacillus sp usually produce more protease during the late exponential phase.
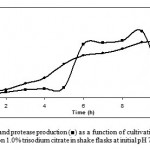 |
Figure 1: Growth (●) and protease production (■) as a function of cultivation time by Bacillus sp KCPSS-3 grown on 1.0% trisodium citrate in shake flasks at initial pH 7.0 and at 50ºC.
|
Bacillus sp. KCPSS-3 was capable of utilizing a wide range of carbon sources. However, the best carbon sources in the present study, for protease production were starch and trisodium citrate, followed by citric acid and sucrose (Table 1).
Table 1: Growth and protease activity by Bacillus sp KCPSS-3 using different carbon sources. The culture density and extracellular protease activity were determined after 9h incubation at 50ºC and initial pH 7.0.
| Carbon source
|
Culture density
(OD 470 nm) |
Protease activity
(U/mg Protein) |
| Glycerol
Galactose Lactose Starch Sucrose Maltose Glucose Casein Trisodium citrate Citric acid |
0.91
0.36 0.37 1.26 0.69 0.21 0.48 0.41 1.04 0.46 |
0.34
0.27 0.27 1.16 0.78 0.43 0.50 0.34 1.13 0.79 |
The type of nitrogen sources also affected the production of protease. Among the various organic and inorganic nitrogen sources, the maximum enzyme activity (1.1 U/mg Protein) was obtained when NH4NO3 was used in the medium (Table 2). Moderate to good levels of enzyme activities were obtained when NH4Cl, KNO3 and ammonium citrate were used as nitrogen sources. When various organic nitrogen sources were tested for protease production, it was found that protease formation by Bacillus sp. KCPSS-3 was repressed, although growth in some cases was stimulated. Similar results were obtained by Ponsare et al. (5) to Aeromonas hydrophila and by Banerjee et al. (1) to Bacillus brevis.
Table 2: Growth and production of protease by Bacillus sp KCPSS-3 using different nitrogen sources. The culture density and extracellular protease activity were determined after 9h incubation at 50ºC and initial pH 7.0.
| Nitrogen source
|
Culture density
(OD 470 nm) |
Protease activity
(U/mg Protein) |
| Peptone (0.5%)
Yeast extract (1.0%) Casein (1.0%) Ammonium citrate (1.0%) Ammonium nitrate (1.0%) Ammonium sulphate (1.0%) Potassium nitrate (1.0%) |
1.5
1.40 0.62 0.57 0.65 0.24 0.54 |
0.24
0.14 0.12 0.72 1.10 0.17 0.57 |
Effect of pH on protease production
The Bacillus sp. KCPSS-3 produced protease over the entire range of pH investigated (pH 5-10). However, the maximum protease production was observed at pH 8.0 while at pH 10, the protease production was about 60% (Fig. 2).
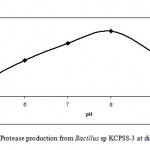 |
Figure 2: Protease production from Bacillus sp KCPSS-3 at different pH.
|
Effect of temperature on protease production
Based on the exhibited proteolytic activity and growth temperature range of 37-70 0C, the effect of temperature on protease production was determined. The Bacillus sp. KCPSS-3 showed maximum protease production at 60 0C. Protease production was about 70% even at 65 0C (Fig. 3). Results reveal that the Bacillus sp under study is a thermophile, and hence, this may be the source of thermostable proteases.
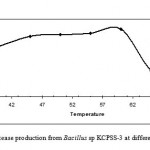 |
Figure 3: Protease production from Bacillus sp KCPSS-3 at different temperatures.
|
Effect of temperature on activity and stability of enzyme
The protease activities were assayed at different temperatures ranging from 37-70 0C at a constant pH of 7.5 (Fig. 4). Enzyme activity increased with temperature within the range of 30 0C to 60 0C. A reduction in enzyme activity was observed at values above 600C. The thermostability.
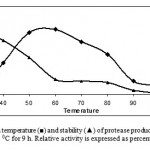 |
Figure 4. Optimum temperature (■) and stability (▲) of protease produced by Bacillus sp KCPSS-3 grown at 50 0C for 9 h. Relative activity is expressed as percentage of the maximum.
|
of the enzyme was examined by measuring the remaining activities at 60 0C, after incubation of the enzyme without substrate at various temperatures between 30 & 90 0C for 2 h. The thermostability profile indicated that the enzyme was stable 30 0C for 2 h while at 40 0C & 80 0C, 14% and 84% of the original activities were lost, respectively.
The results obtained in this study show that there is appreciable high production, activity and stability of the enzyme at high temperatures. This suggests that KCPSS-3 can be a potential producer of extracellular thermostable protease which could find applications in industry and biotechnology. The enzyme thus produced is presently under characterization.
References
- Banerjee, U.C.; Sani, R.K.; Azmi, W.; Soni, R. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Biochem., 35: 213-219, 1999.
- Beg KB, Gupta R (2003) . Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enz. and Microbial Technol., 32: 294 – 304.
- Nunes, A.S.; Martins, M.L.L. Isolation, properties and kinetics of growth of a thermophilic Bacillus. J. Microbiol., 32: 271-275, 2001.
- Pastor, M.D.; Lorda, G.S.; Balatti, A. Protease obtention using Bacillus subtilis 3411 and amaranth seed meal medium at different aeration Braz. J. Microbiol., 32: 1-8, 2001.
- Ponsare, A.C.; Venugopal, V.; Lewis, N.F. A note on nutritional influence on extracellular protease synthesis in Aeromonas hydrophila. Appl. Bacteriol., 58: 101-104.
- Priest, F.G. Extracellular enzyme synthesis in the genus Bacillus. Rev., 41: 711-753, 1977.
- Rao BM, Tanksale MA, Ghatge SM, Deshpande VV (1998). Molecular and Biotechnological Aspects of Microbial Proteases: Microbiol. And Mol. Biol. 62 (3): 597-635.
- Singh, J.; Batra, N.; Sobti, C.R. Serine alkaline protease from a newly isolated Bacillus SSR1. Proc. Biochem., 36: 781-785, 2001.
- Ward, O.P. Proteolytic enzymes. In: M. Moo-Young Editor, Comprehensive Biotechnol., 3: 789-818, 1985.
- Zeikus, J.G.; Vieille, C.; Savchenko, A. Thermozymes: Biotechnology and structure-function relationship. Extremophiles, 1: 2-13, 1998.

This work is licensed under a Creative Commons Attribution 4.0 International License.





