How to Cite | Publication History | PlumX Article Matrix
Huma Ali1*, N. Ganesh1, J. D. Ahi2, Megha Jha1 H. N. Chandel3 and Ashish Maniguha1
1Department of Research Jawaharlal Nehru Cancer Hospital& Research Center, Idgah Hills, Bhopal - 462 001 India.
2Department of Zoology, Dr. Hari Singh Gaur University, Sagar India.
3Department of Pharmacy TRUBA College of Pharmacy Bhopal India.
ABSTRACT: Paracetamol induced a significant elevation of levels of serum marker enzymes; SGOT, SGPT, SALP and total Bilirubin. The 50 % methanolic extract of Ficus carica protects liver tissues against oxidative damages and could be used an effective protector against paracetamol induced hepatic damage. In present study 50% methanolic extract of Ficus carica against paracetamol induced hepatic disorders in mice. Results suggest that the extract possess protective action against hepatic dysfunction induced by the potent toxin, paracetamol. Data showed that the extract responds in both dose and time dependent manner. Administration of 50% methanolic extract of Ficus carica of during paracetamol intoxication prevent the CC14 induced increased levels of serum marker enzymes SGOT, SGPT, SALP and total Bilirubin.
KEYWORDS: Hepatocellular protection; Ficus carica; methanolic leaf extract
Download this article as:| Copy the following to cite this article: Ali H, Ganesh N, Ahi J. D, Jha M, Chandel H. N, Maniguha A. Assessment of Hepatocellular Protection and Cellular Inhibition Potential of Ficus Carica In 50% Methanolic Leaf Extract. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Ali H, Ganesh N, Ahi J. D, Jha M, Chandel H. N, Maniguha A. Assessment of Hepatocellular Protection and Cellular Inhibition Potential of Ficus Carica In 50% Methanolic Leaf Extract. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7271 |
Introduction
Herbal drugs are significant source of hepatoprotective drugs. Mono and polyherbal preparations have been used in various liver disorders. According to one estimate, more than 700 mono and poly herbal preparation in the form of decoction, tincture, tablets and capsules from more than 100 plants are in clinical use. Surprisingly, several studies have appeared in journals addressing hepatotoxic potential of herbal drugs. These studies suggest that the drugs that were claimed to be hepatoprotective are actually hepatotoxic .1
Drug induced liver injury is a potential complication of nearly every medication that is prescribed,2 because the liver is central to the metabolic desposition of virtually of all drugs and foreign substances.3,4
Various scientific approaches have been made to herbal preparations used in Indian system of medicine for the treatment of liver diseases. It has been shown that in addition to the delightful edible fruits, Ficus carica(Fig) leaf extracts are used to lower glucose levels in diabetics and lower the levels of total cholesterol.5 It has been also used as stomachic.5The leaves are added to boiling water and used as a steam bath for painful or swollen piles.5The latex from the stems is used to treat corns, warts and piles.6,7,5 It also has an analgesic effect against insect stings and bites.9The fruit is mildly laxative, demulcent, digestive and pectoral.6,8,5The unripe green fruits are cooked with other foods as a galactogogue and tonic. 5The roasted fruit is emollient and used as a poultice in the treatment of gumboils, dental abscesses etc. 6 Syrup of figs, made from the fruit, is a well-known and effective gentle laxative that is also suitable for the young and very old.9 A decoction of the young branches is an excellent pectoral.8 The plant Ficus carica has anticancer properties. 5 Beyond the treatment of liver disorders, everyday care of the liver lays a cornerstone for total body health. More than 500 vital functions have been identified with the liver. Unfortunately it is extremely difficult to detect early warning symptoms specific to liver metabolic imbalances.
Drug induced hepatotoxicity is a major cause of iatrogenic diseases (drug induced diseases) accounting for 1 in 600 to 1 in 3500 of all hospital admissions and between 2 to 3 percent of all admissions due to reactions. More than 900 compounds including herbal medicines are involved and can produce the full spectrum of liver injuries. Chemicals that cause liver injury are called hepatotoxins. Drug induced liver injury is responsible for 5% of all hospital admissions and 50% of all acute liver failures.10,11
Materials and Methods
Preparation of extract– Fresh leaves were collected locally during the month of June and shade dried for 10 days. About 200gm of powder was obtained from 3kg of leaves. 50% Methanolic extract of dried leaves (ME) was prepared in soxhlet apparatus 22 and concentrated under vacuum using a Speedvac system (SC110A, Savant, USA). The extract was dissolved in double distilled water (DDW, Pyrogen free) freshly before orally administered.
Animals- 24 mice of either sex (Swiss Albino strain) with an average weight of 25±1gm; 6-7 weeks old were used for the experiment. The animals were kept under standard laboratory conditions (day/night rhythm 8:00AM to 8:00 PM, 22˚C room temperature, controlled humidity, standard diet, etc. Altromin pellets and water). The use of animal was as per CPCSEA norms.
Experimental design
The following experiments were conducted: 3
Animals were fasted for 6 hours, before starting the experiment and divided into six groups. Each group contained four animals. The groups were treated as follows.
Group I (Negative Control): Animals were treated with vehicle only (0.20ml of double distilled water) orally for two days. Three doses per day in four hours interval were given to each animal in this group.
Group II (Positive Control): Animals were treated with vehicle (0.20ml of double distilled water) orally for two days (three doses per day in four hours interval). After two hours of first administration as vehicle, the animals were treated with 500mg/kg. Acetaminophen i.p.
Group III (Standard Group): Animals were treated with 50mg/kg. Silymarin orally for two days three doses per day in four hours interval). The Silymarin stock solution was prepared in double distilled water. After two hours of first administration of silymarin, the animals were treated with 500mg/kg acetaminophen i.p.
Group IV (Test Group A): Animals were treated with 125 mg/kg. Methanolic extract of drug in double distilled water orally for two days (three doses per day in four hours interval). After two hours of first administration of methanolic extract, the animals were treated with 500mg/kg acetaminophen i.p.
Acetaminophen solution for i.p.(intra peritoneal) administration was prepared from Neomol injection, Neon laboratories Ltd, Thane Maharashtra, and silymarin solution for oral administration was prepared from silybon 140 Tab,. Microlab Ltd., Goa.
All animals were sacrificed after 48 hours of acetaminophen administration. Whole blood from the carotid artery of animal was drawn for assessment of liver function. Liver tissue of animal was removed, flushed with normal saline and fixed in 10% neutral formalin solution for histopathological evaluation.
Analytical Methods
Estimation of Serum Alanine Aminotransferase (SGPT or S. ALP) (Kinetic method). 12,13 Thus, SGPT concentration of sample was determined in IU/L.
Estimation of Serum Aspartate Aminotransferase (SGOT or S. AST). 12 Thus, SGOT concentration of sample was determined in IU/L.
Estimation of Serum Alkaline phosphatase (S. ALP).14,15 Thus, S. ALP concentration of sample was determined in IU/L.
Estimation of total Bilirubin (Jendrassik & Grof Method). 16,17,18 Thus, total bilirubin concentration of sample was determined in mg/dl.
Histopathological Evaluation
After blood collection, the blood samples were left to coagulate at room temperature for 1 hour. Serum was separated by centrifugation at 3000 rpm and 4˚C for 20 minutes. The serum was tested for SGOT, SGPT, Alkaline phosphate and bilirubin content.
Obseravation and Results
The effect of 50% methanolic extract of Ficus carica on SGOT, SGPT, S.ALP and Total Bilirubin is in paracetamol induced liver damage in mice are summarized in table -1.Administration of paracetamol (500mg/kg; body weight), resulted a significant elevation of hepatospecific serum markers SGOT, SGPT, SALP and total Bilirubin in paracetamol treated group, in comparison with the normal control group. On administration of crude extract of Ficus carica at different doses of 125mg/kg ,250mg/kg and 500mg/kg body weight and Silymarin at the dose of 25mg/kg, the level of these enzymes were found retrieving towards normalcy.
The level of SGOT is when compared with positive control; it was found that 500mg/kg of crude extract of Ficus carica have shown one fold lower SGOT value. However standard group have shown averagely better result when the test group. All the test doses have found decrease SGOT values when compared with positive control group. there is no significant change in values of SGPT in group A and C . Group B have shown better response rate then the positive control. The ALP level is significantly decreased in group C then standard group. Group A, B, and C have shown mine to moderate decease in total Bilirubin levels (Graph 1-4)
The results on the effects of Ficus carica methanolic extract on serum enzyme level (SGOT, SGPT, ALP and Total Bilirubin) in mice with liver-injury induced by Paracetamol are summarized in Table 1. It is well known that Paracetamol is used as a hepatotoxic agent on various animals 19,20. Administration of Paracetamol to rats produced hepatotoxicity showed by significant increase in the serum levels of ALT, AST, ALP and Total bilirubin in comparison to positive control group. The methanolic extract of Ficus carica showed a significant decrease in the serum enzymes ALT, AST, ALP and Total bilirubin as compared to positive control.
Table 1: Effect of methanolic extract of plant Ficus carica on serum enzyme level in mice.
| Group | SGOT
(S.AST) in IU/L |
SGPT
(S.ALP) in IU/L |
Alkaline phosphate (ALP) in IU/L | Total Bilirubin in mg/dl |
| Positive control group
(PCM+vehicle) |
159.50 ± 7.23 |
257.59 ± 5.23 |
518.13 ± 6.74 |
0.4025± 0.031 |
| Negative control group
(vehicle only) |
36.07 ± 6.53 |
100.43 ± 9.96 |
344.05 ± 7.43 |
0.2225 ± 0.011 |
| Standard group (Sylmarin 50mg/kg orally) |
70 ± 6.02 |
163.28 ± 6.44 |
371.63± 5.71 |
0.2725± 0.017 |
| Test group A (Methanolic extract 125mg/kg orally) |
97.45 ± 9.16 |
217.33± 5.84 |
386.43 ± 20.44 |
0.3925 ± 0.006 |
| Test group B (Methanolic extract 250mg/kg orally) |
79.40± 5.14 |
196.5 |
367.81 ± 11.02 |
0.3825± 0.041 |
| Test group C (Methanolic extract 500mg/kg orally) |
73.51 ± 3.98 |
203.35 ± 15.6 |
359.63 ± 13.74 |
0.355 ± 0.041 |
GRAPHS
 |
Graph 1.1: Effect of Methanolic leaf extract of Ficus carica on SGOT Level of mice
|
 |
Graph 1.2: Effect of Methanolic leaf extract of Ficus carica on SGPT Level of mice.
|
 |
Graph 1.3: Effect of Methanolic leaf extract of on Ficus carica.
|
Alkaline Phosphate Level of mice
 |
Graph 1.4: Effect of Methanolic leaf extract of Ficus carica on .Total Bilirubin Level of mice
|
Graph 1.3: Effect of Methanolic extract of Ficus carica on
Alkaline Phosphate Level of mice
PHOTOGRAPH : HISTOPATHOLOGICAL STUDY OF HEPATOPROTECTIVE ACTIVITY
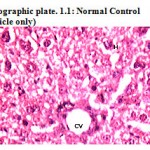 |
Photographic plate 1.1: Normal Control (vehicle only)
|
T.S. of liver revealed norm chromatic hepatocytes, occasionally apoptosis, cart wheel nucleus and lipid vacuoles. A good mitotic index has been observed.
BD – Bile Duct
H – Hepatocyte
CV – Central vein
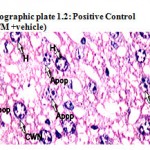 |
Photographic plate 1.2: Positive Control (PCM +vehicle).
|
T.S. of liver revealed hyper chromatic and highly damaged hepatocytes. Large numbers of lipid vacuoles have been observed which propelled nucleus to the periphery of the cell. Frequent apoptosis and necrosis with poor mitotic index have been observed.
Apop – Apoptosis
CWN – Cart wheel Nucleus
H – Hepatocyte
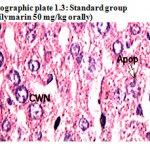 |
Photographic plate 1.3: Standard group (Silymarin 50 mg/kg orally).
|
T.S. of liver revealed normochromatic Hepatocyte. Occasionally apoptosis, cart wheel nucleus and lipid vacuoles have been observed with average mitotic index.
Apop – Apoptosis
CWN – Cart wheel Nucleus
 |
Photograph 2: Histopathological Study Of Hepatoprotective Activity
|
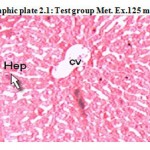
Photographic plate 2.1: Test group Met. Ex.125 mg/kg
T.S. of liver revealed normochromatic hepatocytes with good mitotic index. Occasionally lipid vacuoles and apoptosis have been observed without necrosis and cart wheel nucleus.
Hep – Hepatocyte
CV – Central vein
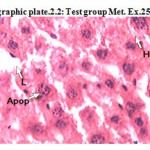 |
Photographic plate.2.2: Test group Met. Ex.250mg/kg
|
T.S. of liver revealed normochromatic hepatocytes with good mitotic index. Occasionally lipid vacuoles have been observed with apoptosis,no necrosis and cart wheel nucleus were found
Apop – Apoptosis
L – Lipid vacules
H – Hepatocyte
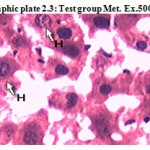 |
Photographic plate 2.3: Test group Met. Ex.500 mg/kg.
|
T.S. of liver revealed normochromatic hepatocytes with good mitotic index.
No apoptosis, necrosis and cart wheel nucleus were observed in this group.
H – Hepatocyte
References
- Ostapowicz G. Fontana R. J., Schiodt F.V., “Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States”. Ann. Intern .Med. 2002; 12:947-54.
- Zimmerman H J, Maddrey WC. Toxic and drug induced Hepatitis. In: Schiff L, Schiff ER, eds. Diseases of the liver. Philadelphia : J.B. Lippincott, 1993.
- Kaplowitz N. Drug metabolism and hepatotoxicity . In: Kaplowitz N, ed. Liver and billary diseases. Baltimore : Williams and Wilkins, 1991.
- Farrell GG Drug – induced liver disease .Edinburgh, Scottland; Churchill Livingstone, 1994
- Duke. J. A. and Ayensu. E. S. Medicinal Plants of China Reference Publications, Inc.1985 ISBN 0-917256-20-4 Grieve. A Modern Herbal. Penguin 1984 ISBN 0-14-046-440-9
- Polunin. O. and Huxley. A. Flowers of the Mediterranean. Hogarth Press 1987 ISBN 0-7012-0784-1
- Chiej. R. Encyclopaedia of Medicinal Plants. MacDonald 1984 ISBN 0-356-10541-5
- Chevallier. A. The Encyclopedia of Medicinal Plants Dorling Kindersley. London 1996 ISBN9-780751-303148
- Peter F. GI/Liver Secrets: with STUDENT CONSULT Access. Saint Louis: C.V. Mosby. cNally
- Ostapowicz G, Fontana RJ, Schiødt FV, et al (2002). “Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States”. Ann. Intern. Med. 137 (12): 947–54.
- Clin. Clim. Acta. 105, 147-172 (1980)
- Thefeld,w. et .al., Dtsch. Med. Wschr.99, 343 (1994)
- Bretandiere, J.P. et al., Clin. Chem. 23:2263 (1977)
- Bowers, G.N. et al ., Clin. Chem. 21:1995 (1976)
- Jendrassik L and Grof.P. Biochem Z.297, 81 (1938)
- Schellong.G and Wende.U. arch Kinderheik 162,120 (1960)
- Gambino.S.R., in “Standard Methods of Clinical Chemistry”,S.Meites Ed.,Academic Press. Vol. 5(1965) P.55.
- Johnson DE, Kroening C. Pharmacol Toxicol 1998; 83:231.
- Recknagel RO. Trends Pharmacol Sci 1983; 4:129.
- Suffness M. & Douros J, Drugs of plant origin, in: Methods in Cancer research, edited by VT de Vita, Vol. 16 (Academic press Inc, New York)1979, 73.

This work is licensed under a Creative Commons Attribution 4.0 International License.





