How to Cite | Publication History | PlumX Article Matrix
Development and Characterization of Mucoadhesive Microspheres of Ofloxacin
Ripudaman Singh1*, R. Irchhaiya, Manjulata, R.K. Arya and A.K. Gupta
Institute of Pharmacy, Bundelkhand University, Jhansi - 284128 India.
ABSTRACT: A series of mucoadhesive microspheres of ofloxacin have been successfully prepared, using chitosan as a polymer cross-linked with glutaraldehyde as the shell. The morphology was characterized by scanning electron microscopy (SEM). The swelling and releasing behaviors of the microspheres at pH 1.2 were investigated. The results revealed that microsphere exhibited very smooth and spherical surface and electrostatic interaction existed between cross-linked chitosan and ofloxacin. Ofloxacin bounded in the microspheres was slowly released in 0.1N HCl (pH 1.2) because the degree of swelling of the microsphere was very low. The release mechanism of the mucoadhesive microsphere was through the swollen microspheres and to be controlled by the cross-linking shell density of the microspheres. The higher the cross-linking density of, the lower is the swelling ability of the chitosan microsphere due to the slower relaxation of polymer chain, which then results in decreased drug release rate.
KEYWORDS: Mucoadhesive; microspheres; ofloxacin
Download this article as:| Copy the following to cite this article: Singh R, Irchhaiya R, Manjulata, Arya R. K, Gupta A. K. Development and characterization of mucoadhesive microspheres of ofloxacin. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Singh R, Irchhaiya R, Manjulata, Arya R. K, Gupta A. K. Development and characterization of mucoadhesive microspheres of ofloxacin. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7503 |
Introduction
Oral sustained release dosage forms are the most commonly formulated but still offer highest attention in the area of novel drug delivery systems.1 One of the novel approaches in this area is mucoadhesive delivery system. The mucoadhesive controlled release formulations have gained the considerable attention due to their ability to adhere to the mucus layer and release the loaded drug in a sustained manner. By using these dosage forms, intimate contact time with the mucus surface would increase, which results in an increased drug retention at the site, which leads to the improved therapeutic efficacy for the treatment of gastrointestinal tract infection.2 Recent studies prove that chitosan has mucoadhesive properties and therefore it seems particularly useful to formulate bioadhesive dosage forms for mucosal administration (ocular, nasal, buccal, gastro-enteric and vaginal-uterine therapy).3 Chitosan, poly[b-(1-4)-linked-2-amino-2-deoxy-o-glucose], is produced from chitin which is naturally abundant in the marine crustacean. Chitosan could be cross-linked using glutaraldehyde.4 Mucoadhesive microspheres include microparicles and microcapsules(having a core of the drug) of 1-1000µm, in diameter and consisting either entirely o a mucoadhesive polymer or having an outer coating of it, respectively5.
Ofloxacin is the drug whose bioavailability is strongly dependent on the local physiology in the GI tract and which preferably is absorbed in higher section of intestine. Ofloxacin is readily soluble in acidic environment of the stomach. In the intestine, where neutral to slightly alkaline pH conditions prevail; however, precipitation of the active compound occurs, which adversely affects absorption in the lower sections of intestine. There is a need for system that reside in stomach over a relatively long time and release the active compound there in sustained manner6. This necessitated the design and development of mucoadhesive drug delivery system for ofloxacin using chitosan as a polymers. The objective of present work was to develop mucoadhesive formulation which releases drug in stomach and upper gastrointestinal (GI) tract, and form an enhance opportunity of absorption in the stomach and upper GI tract rather than the lower portion of GI tract.
Material and Methods
Ofloxacin was obtained as gift sample from Finecure pvt. Ltd. (Uttarakhand, India). Chitosan was obtained as gift sample from Central Institute of Fisheries Technology (Cochin, India). Dioctyl Sodium Sulphosuccinate (DOSS) and Petroleum Ether 80:20 were procured from (CDH, New Delhi). Liquid Paraffin from (Merck, Mumbai) and Glutaraldehyde was purchased (CDH, New Delhi). All chemicals used were of AR grade.
Methods
Preparation of Microspheres
Mucoadhesive microspheres of chitosan were prepared by simple emulsification phase separation technique. Chitosan was used as a polymer and was cross-linked using glutaraldehyde as per method as per method described by Thanoo et al.7
Chitosan (100mg) was dissolved in 10ml of 1% v/v aqueous acetic acid solution. 100mg of drug was dispersed in the polymer solution. In batches CO-A to CO-F the polymer-to-drug ratio was kept constant at 3:1. The resultant mixture was extruded through a syringe in 1L of liquid paraffin (heavy and light, 1:1 ratio) containing 0.2% DOSS, and stirring was performed using a propeller stirrer (Remi, Mumbai, India). After 15 minutes, glutaraldehyde (25% v/v aqueous solution) was added and stirring was continued. The amount of cross-linking agent and cross-linking time were varied in batches CO-A to CO-F from 2.5 to 15 ml and 1 to 3 hours, respectively as shown in Table 1. In batches CO-1 to CO-4, 10 ml of glutaraldehyde was used as a cross-linking agent and cross-linking time was kept to 1 hour. The polymer-to-drug ratio and stirring speed were varied in batches CO-1 to CO-4 as shown in Table 2. All other variables were used as mentioned in preliminary trial batches. Microspheres thus obtained were filtered and washed several times with petroleum ether (80:20) to remove traces of oil and glutaraldehyde. The microspheres were then dried at room temperature (at 250c) for 24hours. The effect of formulation variables on characteristics of the microspheres in summarized in Tables 1 and 2.
Drug Entrapment Efficiency
50 mg of dried microsphere were weighted accurately and were crushed in a glass mortar pestle and the powdered microspheres were suspended in 10 ml of 0.1 N HCl (pH-1.2). After 24 hrs the solution was filtered and the filtrate was analyzed for the drug content. Entrapment efficiency was calculated according to equation.8
Entrapment efficiency = (Practical drug content/Theoretical drug content) × 100
The result is summarized in the Tables 1 and 2.
Mucoadhesion study
The mucoadhesive properties of the microspheres were evaluated by in vitro wash-off test as reported by Lehr et al.9 A 1 cm piece of rat stomach mucosa was tied onto a glass slide (3 inch by 1 inch) using thread. Microspheres were spread (100) onto the wet rinsed tissue specimen and the prepared slide was hung onto one of the groves of a USP tablet disintegrating test apparatus. The disintegration test apparatus was operated such that the tissue specimen was given regular up and down movement in a beaker containing the simulated gastric fluid USP (pH 1.2). At the end of sixty minutes and hourly intervals upto 10 hrs., the no. of microspheres still adhering onto the tissue was counted. The results of in vitro wash-off test of batches CO-A to CO-F and CO-1 to CO-4 are shown in tables 1 and 4 respectively.
Particle size of microspheres
The particle size of the microspheres was determined by using optical microscopy method.10 Approximately 100 microspheres were counted for particle size using a calibrated optical microscope. The observed data is reported in tables 1 and 2.
Table 1: Evaluation of preliminary trial batches.
| Batch
|
Polymer
Volume of Crosslinking |
% | % Drug | Particle | |
| No.
|
to drug
crosslinking |
time (hrs.) Mucoadhesion entrapment |
|
size (µm ± | |
|
(ml) |
ratio agent
|
after 1 hr. | efficiency | S. D.) | |
| CO-A | 3:1 2.5 | 1 | 81.12 | 71.02 | 49.81±1.35 |
| CO-B | 3:1 5 | 2 | 78.43 | 74.46 | 41.88±1.09 |
| CO-C | 3:1 7.5 | 3 | 74.21 | 76.13 | 27.24±1.62 |
| CO-D | 3:1 10 | 1 | 75.85 | 82.81 | 25.58±0.76 |
| CO-E | 3:1 12.5 | 2 | 68.28 | 81.28 | 20.34±2.19 |
| CO-F | 3:1 15 | 3 | 65.33 | 84.11 | 18.56±3.21 |
All batches were prepared at polymer to drug ratio 3:1
Table 2: Evaluation of drug entrapment efficiency, particle size and drug release of the microspheres.
| Batch
|
Polymer
Volume of Crosslinking |
% | % Drug | Particle | |
| No.
|
to drug
crosslinking |
time (hrs.) Mucoadhesion entrapment |
|
size (µm ± | |
|
(ml) |
ratio agent
|
after 1 hr. | efficiency | S. D.) | |
| CO-1 | 1:1 10 | 1 | 63.82 | 12.83±2.44 | 84.64±1.75 |
| CO-2 | 2:1 10 | 1 | 72.25 | 20.81±1.78 | 81.93±1.37 |
| CO-3 | 3:1 10 | 1 | 81.93 | 25.51±0.97 | 76.21±1.93 |
| CO-4
|
4:1 10
|
1
|
79.54
|
75.98±1.57 | 40.65±1.17 |
Scanning electron microscopy
Scanning electron photomicrographs of drug-loaded chitosan microspheres were taken. A small amount of microspheres was spread on glass stub. Afterwards, the stub containing the sample was placed in the scanning electron microscope (PHILIP 505) chamber. The scanning electron photomicrograph was taken at the acceleration voltage of 14.9 kV. The photomicrographs are depicted in Figure 1.
Swelling index of microspheres
For estimating the swelling index, the microspheres (100) were suspended in 5 ml of simulated gastric fluid USP (pH 1.2).11 The particle size was monitored by microscopy technique every 1 hour using an optical microscope. The increase in particle size of the microspheres was noted for up to 10 hours, and the swelling index was calculated as per method described by Ibrahim.12 The swelling index for microspheres of batches CO-1 to CO-4 is reported in Table 3.
Table 3: Swelling index of mucoadhesive microspheres.
| Time (hrs.) | 4
Swelling index ± S.D. |
||||
| Time (hrs.) | 1 | 2 | 4 | 8 | 10 |
| CO-1 | 0.370±0.13 | 0.486±0.45 | 0.673±0.26 | 0.911±0.10 | 0.988±0.19 |
| CO-2 | 0.314±0.22 | 0.436±0.34 | 0.634±0.31 | 0.996±0.20 | 1.039±0.23 |
| CO-3 | 0.286±0.19 | 0.352±0.25 | 0.599±0.15 | 0.949±0.25 | 1.180±0.20 |
| CO-4 | 0.159±0.21 | 0.287±0.22 | 0.465±0.18 | 0.935±0.22 | 1.206±0.19 |
Drug release study
The drug release study was performed using USP XXIV basket apparatus at 37°C ± 0.5°C and at 50 rpm using 900 ml of 0.1N HCl (pH 1.2) as a dissolution medium (n = 3) as per USP XXVI dissolution test prescribed for ofloxacin extended release tablets. Microspheres equivalent to 100 mg of ofloxacin were used for the test. 5 ml of sample solution was withdrawn at predetermined time intervals, diluted suitably, and analyzed spectrophotometrically. An equal amount of fresh dissolution medium was replaced immediately after withdrawal of the test sample. Percentage drug dissolved at different time intervals was calculated using the Lambert-Beer’s equation (y = 0.067x – 0.000, R2 = 0.999) described above. The percentage drug release of batches CO-1 to CO-4 is shown in figure 2.
Table 4: Results of in-vitro off test to access the mucoadhesion of the microspheres.
| Batch No. % Mucoadhesion to stomach mucosa ± S.D | |||||
| Time (hrs.) | 1 | 2 | 4 | 8 | 10 |
| CO-1 | 80±1.28 | 74±1.23 | 64±1.55 | 44±1.43 | 34±3.23 |
| CO-2 | 83±1.76 | 75±1.44 | 66±2.36 | 47±1.58 | 38±1.34 |
| CO-3 | 85±1.45 | 74±2.31 | 65±1.53 | 46±1.76 | 39±1.32 |
| CO-4 | 84±1.23 | 73±1.22 | 63±2.03 | 42±1.56 | 31±1.94 |
Results and Discussion
The mucoadhesive microspheres of chitosan were prepared by simple emulsification phase separation technique. Chitosan was selected as a polymer for the preparation of mucoadhesive microspheres due to its biodegradable and mucoadhesive properties. The formulation of the present microspheres is based on the solubility behavior of chitosan, which is poorly soluble in water. In the presence of acetic acid, chitosan shows good aqueous solubility. Different concentrations of acetic acid from 1% w/v to 6% w/v were tried but no significant effect of acetic acid was observed on % mucoadhesion or drug entrapment efficiency, therefore 1% w/v of acetic acid was selected for the study.
One of the important factors related to microspheres is viscosity of the polymer solution. Polymer concentrations of 0.5%, 1% and 2% w/v were selected for preliminary trials. Flake formation was observed when chitosan concentration was used at a level of 0.5% whereas maximum sphericity was observed at 1% level. The chitosan solution was found to be a too viscous to pass through the syringe when use at 2% level. Therefore 1% w/v of chitosan in 1% acetic acid was found to be the optimum concentration. The addition of 0.2% w/v of DOSS to the dispersion medium was found to be essential to minimize aggregation of microspheres.
Preliminary trials were carried out to optimize the process of preparation. Batches CO-A to CO-F were prepared to study the effect of volume of cross-linking agent (glutaraldehyde), time for cross-linking and stirring speed on the % mucoadhesion, drug entrapment efficiency and particle size.
The volume of glutaraldehyde was varied from 2.5, 5, 7.5, 10, 12.5 and 15 ml. Discrete spherical microspheres (Batches CO-D to CO-F) were obtained using 10, 12.5 and 15 ml of glutaraldehyde. Batches CO-A to CO-C prepared using 2.5, 5, 7.5 ml yielded irregular microspheres. The higher amount of glutaraldehyde appears to favor the cross-linking reaction and hence spherical free flowing microspheres were obtained. The microspheres of batches CO-D to CO-F also showed significant effect on the % mucoadhesion and drug entrapment efficiency. Batches CO-A to CO-C prepared using 2.5, 5, 7.5 ml glutaraldehyde showed good % mucoadhesion but drug entrapment efficiency was below 75 %. Batches CO-D to CO-F also showed good mucoadhesion as well as 75 % drug entrapment efficiency. In the microspheres of batches CO-E and CO-F the drug entrapment efficiency was above 75% but mucoadhesion decreased. Thus, we can conclude that 10 ml of glutaraldehyde was the optimum amount.
At the end of 60 min (1 hr) the percent mucoadhesion was found 81.12, 78.43, 74.21, 75.85, 68.28 and 65.33 for the formulations CO-A, CO-B, CO-C, CO-D, CO-E and CO-F, respectively. Increase in the cross-linking time (1 to 3h) inversely affected the % mucoadhesion. The cross-linking polymer probably becomes more rigid and thus mucoadhesiveness decreases. The cross-linking time did not have a significant effect on the drug entrapment efficiency.
To investigate the effect of stirring speed, batches were prepared at 500 and 1000 rpm. The results were in general agreement with general theory of microspheres that the particle size of the microspheres prepared at 1000 rpm was smaller than those prepared at 500 rpm. Increase in the volume of glutaraldehyde (10 to 15 ml) slightly increases the particle size as seen in size comparison of batches. Comparison of batches CO-D and CO-F prepared at same speed reveals that the cross-linking time did not have a significant effect on particle size. The microspheres of batches CO-A to CO-C were irregular and having the particle size between 27 – 50 µm. Moreover microspheres of batches CO-D to CO-F had a particle size below 25 ìm and it was between 12.83 µm, 20.81 µm, 25.51 µm and 40.65 µm for the formulations CO-1, CO-2, CO-3 and CO-4 respectively.
Drug entrapment efficiency was observed maximum 84.11% in CO-F, 82.81% in CO-D, 81.28 in CO-E, 76.13 in CO-C, 74.46 in CO-B and 71.02 in CO-A, where the polymer to drug ratio was 3:1 for all the formulations. This was because the chitosan showed good entrapment efficiency due to its cross-linking with the glutaraldehyde which leads to the formation of an outer shell of microspheres and closely binds the drug molecules with the polymer backbone.
On the basis of above studies further four batches were prepared using 10 ml of glutaraldehyde; the cross-linking time was kept at 1 hr and speed 1000 rpm. Characterization of the formulations was done on the basis of polymer to drug ratio. The polymer to drug ratio was kept 1:1, 2:1, 3:1 and 4:1 for the formulations CO-1, CO-2, CO-3 and CO-4 and effect were studied on the characteristics of microspheres. Effect of changing the polymer to drug ratio on various process parameters such as mucoadhesion, entrapment efficiency, particle size and surface morphology, swelling index and drug release were studied and by considering the observation, the results are discussed here.
On increasing the concentration of chitosan, the amount of drug entrapment was increased as, it was observed maximum 81.93% in CO-3 and minimum 63.82% in CO-1 where the polymer to drug ratio was 3:1 and 1:1, respectively. The particle size of the microspheres was 12.83 µm, 20.81 µm, 25.51 µm and 40.65 µm for the formulations CO-1, CO-2, CO-3 and CO-4 respectively, the stirring speed was 1000 rpm.
Surface morphology of the microspheres was examined by scanning electron microscopy (SEM). It was observed that surface of the microspheres was very smooth and spherical in shape. The formulation CO-3 taken for scanning electron microscopic analysis of microspheres was observed, as shown in figures 1
 |
Figure 1: Scanning electron micrographs of the formulation CO-3 at the acceleration voltage of 14.9 kv. Showing smooth and spherical surface of microspheres.
|
Swelling studies data reveal that the amount of polymer and pH of the medium play an important role in drug release. The microspheres in 0.1 N HCl have a lower percentage degree; therefore, the swelling ability of the microspheres is weakened in the acid environment. It can be concluded from data shown that with an increase in polymer concentration, the time for maximum swelling index increase. Thus, we can conclude that amount of polymer directly affects the swelling index.
To assess the in-vitro mucoadhesion of the microspheres in-vitro wash off test was performed for all the formulations. At the end of 600 min (10 hrs) the percent mucoadhesion was found 34, 38, 39 and 31 for formulation CO-1, CO-2, CO-3 and CO-4, respectively, shown in table 25. Formulation CO-3 showed the highest mucoadhesion due to the presence of higher proportion of chitosan, due to the cationic nature of the polymer, and CO-1 showed the lowest mucoadhesion due to lower proportion of chitosan which lead to less free NH2 groups to bind with Sialic groups present on mucin layer.
The drug release studies show the effect of environment on the drug release pattern of the prepared microspheres. The in-vitro release was observed in 0.1 N HCl (pH 1.2) for 12 hrs. It was found that the release rate from the all formulation was found to be different for the different polymer proportion used in the formulation. The amount of drug released in 12 hrs. was 84.64%, 81.93%, 76.21% and 75.98.0% for the formulations CO-1, CO-2, CO-3, and CO-4, respectively. The CO-4 having the highest proportion of polymer chitosan, showed minimum release. While the CO-3 showed the drug release of 76.21% after 12 hrs due to higher swelling of the microspheres.
The release curve suggests that the drug is included inside the cross-linked shell because only less than 11% ofloxacin is released from the microspheres within 1 hour. It was the case of release from the surface, most of the adsorbed drug is released within 45 min. when the microspheres come in the contact with the release medium, leading to the burst or disrupt in microsphere effect in the early stage of dissolution. This can be attributed to the both, existence of stronger electrostatic interaction and stability of the cross-linked shell in acidic medium. The drug release in 4 hours was less than 40%. This suggests the higher density of the cross-linked shell due to which the electrostatic interaction of the microspheres was not easily broken at acidic pH. This was because of the formation of cross-linked shell by the reaction between glutaraldehyde and chitosan. At the end of 4 hour the drug release from the microspheres increases, because of the increase in the degree of swelling of microspheres due to which the cross-linked density decreases, leading to a loose structure shell and release of the drug from the microspheres occurs. This further confirms the existence of the drug in cross-linked shell. If the cross-linked shell has not been cross-linked completely, ofloxacin easily diffuses from the microspheres on account of the loose structure. The drug release data is reported in the figure 2.
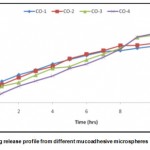 |
Figure 2: In vitro drug release profile from different mucoadhesive microspheres Click here to View figure |
The drug release from batches CO-1 to CO-4 indicates that as the polymer to drug ratio is increased, the time to release the drug decreases. Batch CO-4 showed minimum drug release in 12 hrs. However, batch CO-4 showed low % mucoadhesion as compared to batches CO-3. Batch CO-3 prepared at 3:1 polymer to drug ratio showed highest that is 84.15 % mucoadhesion after 1h, 81.93 % drug entrapment efficiency and good swelling properties.
The particle size of microspheres of batch CO-3 is 25.51 µm. There is no significant difference between the cumulative % drug release of batches CO-3 and CO-4 but good mucoadhesion and drug entrapment was observed in batch CO-3 as compared to the batch CO-4, Thus, batch CO-3 was considered as the best batch. Thus, we can conclude that Ofloxacin release from the mucoadhesive microspheres was slow and extended and it depended on the processing parameters and polymer to drug ratio.
The study demonstrates the preparation of mucoadhesive microspheres of Ofloxacin using chitosan as a polymer. Glutaraldehyde was used as a cross-linking agent. The volume of cross-linking agent, polymer to drug ratio and stirring speed had a significant effect on the microspheres characteristics. The microspheres also showed good mucoadhesive properties and were able to give extended drug release of Ofloxacin
Acknowledgements
The authors are thankful to The Head, Institute of Pharmacy, Bundelkhand University, Jhansi for encouragement and providing the necessary facilities to carry out the research work, CDRI Lucknow for providing IR spectral data and BSIP Lucknow for providing SEM facility.
References
- Chavanpatil M.D., Jain P., Chaudhary S., Shear R., Vavia P.R., Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for Ofloxacin, Int. J. Pharm., 316: 86-92 (2006).
- Yadav K.S.M., Satish C.S. and Shivakumar H.G., Preparation and evaluation of chitosan-poly (acrylic acid) hydrogels as stomach specific delivery for amoxicillin and metronidazole, Indian J. Pharm. Sci., 69(1): 91-95 (2007).
- Genta I., Constantini M., Asti A., Conti B., Montanari L., Influence of glutaraldehyde on drug release and mucoadhesive properties of chitosan microspheres, Carbohydrate polymers, 36: 81-88 (1998).
- Mi F.-L., Kuan C.-Y., Shyu S.-S., Lee S.-T., Chang S.-F., The study of gelation kinetics and chain relaxation properties of glutaraldehyde cross linked chitosan gel and their effects on microspheres preparation and drug release, Carbohydrate polymers, 41: 389-396 (2000).
- Chowdary K.P.R. and Rao Y.S., Mucoadhesive microspheres and microcapsules: Current status, Indian J. Pharm. Sci., 67(2): 141-150 (2005).
- Chavanpatil M.D., Jain P., Chaudhary S., Shear R., Vavia P.R., Development of sustained release gastroretentive drug delivery system for Ofloxacin: In vitro and
in vivo evaluation, Int. J. Pharm., 304:
178-184 (2005). - Thanoo B.C., Sunny M.C., Jayakrishnan A., Cross linked chitosan microspheres: Preparation and evaluation as a matrix for the controlled release of the pharmaceuticals, J. Pharm. Pharmacol., 44: 283-286 (1992).
- Patel J.K., Bodar M.S., Amin A.F. and
Patel M.M., Formulation and optimization
of mucoadhesive microspheres of metoclopramide, Indian J. Pharm. Sci.,
66(3): 300-305 (2004). - Lehr C.M., Bowstra J.A., Tukker J.J., JungingerH.E., Intestinal transit of bioadhesive microspheres in an in situ loop in the rat, J. Control release, 13: 51-62
(1990). - Milling E.L., Lachman L., Liberman H.A., Eds., The theory and practice of industrial pharmacy, 2nded., Mumbai, India, Varghese publishing house, 26-27 (1991).
- United States Pharmacopeial Convention, XXVI, In:The united states pharmacopeia, Rockville, MD, United States Pharmacopeial Convention, Inc; 2528 (2003).
- El-Gibaly I., Development and in vitro evaluation of novel floating chitosan microcapsules for oral use: Comparison with non floating chitosan microspheres, Int. J. Pharm, 249: 7-21 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.





