How to Cite | Publication History | PlumX Article Matrix
R. K. M. Mutahar1*, C. Nagesh2, T. V. Narayana1, S. C. Marihal1, R. Ramesh1 and S. M. nizami1
1P.G. Department of Pharmaceutics and Pharmacology. Dr. H.L.T.College of Pharmacy, Kengal, Channapatna, Bangalore (Rural) - 571 502 India. 2P.G. Department of Pharmaceutics, S.C.S.College of Pharmacy, Harapanahalli India.
ABSTRACT: In the present research, an attempt has been made to formulate controlled release matrix tablets of nicotinic acid (NA), using xyloglucon (XGL), xanthan gum and guar gum separately, which tend to reduce “flushing” effect caused by immediate release of NA. Tablets were evaluated for uniformity of weight, content of active ingredient, friability, hardness, thickness, in vitro dissolution and swelling index. All the formulations showed compliance with pharmacopoeial standards. After hydration of gum, drug release was essentially pH-independent. The amount released was directly proportional to the loading of dose and inversely proportional to gum concentration in tablets. Selected formulations were subject to stability studies, which showed stability with respect to release pattern. It can be concluded that, apart from guar gum, and xanthan gum the XGL can be successfully used as an effective matrix material to retard the release of NA for extended period of time.
KEYWORDS: Nicotinic acid; Controlled release; Hydrophilic polymers; Xyloglucon; Gaur gum; Xanthan gum
Download this article as:| Copy the following to cite this article: Mutahar R.K.M, Nagesh C , Narayana T. V, Marihal S. C, Ramesh R and Nizami S. M. Formulation and in vitro evaluation of controlled release matrix tablets of nicotinic acid using xyloglucon and other hydrphilic polymers. Biosci Biotechnol Res Asia 2008;5(2). |
Introduction
Hydrophilic matrices are an interesting option when developing an oral sustained-release formulation. They can be used for controlled release (CR) of both water-soluble and water-insoluble drugs. The release behavior of drugs varies with the nature of the matrix and is a complex interaction of swelling, diffusion and erosion process1. Release of drug from such matrices can be controlled through: their physical properties, the correct choice of gelling agent and setting up the conditions for fabrication2.
Matrix system is the most innumerable method used in the development of sustained release formulations. It is the release system, which prolongs and controls the release of drug that is dissolved or dispersed. In fact, matrix is defined as a well composite of one or more drugs with a gelling agent i.e. hydrophilic polymer.3
Polysaccharides are the choice of materials among the hydrophilic polymers used, because they are nontoxic and acceptable by the regulating authorities 4 The various polysaccharides used in drug delivery application are cellulose ethers,5 xanthan gum,3 scleroglucan,6 locust bean gum,7 and gaur gum 8 etc. Xanthan gum hydrophilic polymer, that not only retards the drug release but also provides the time independent release kinetics with advantages of biocompatibility and inertness 9. Guar gum has been investigated as CR carrier and regarded as nontoxic and nonirritant material 10.
Another natural polysaccharide xyloglucon obtained from the seed kernel of Tamarindus indica, possesses properties like high viscosity, broad pH tolerance, 11 non carcinogenic, 12 mucoadhesive, and biocompatible 13 It is used as stabilizer, thickener, gelling agent, and binder in food and pharmaceutical industries. The tamarind seed polysaccharide, obtained from kernels of tamarind tree, is indigenous to India and South East Asia, constituting about 65% of the seed components.14 ,15 lt is a branched polysaccharide with a main chain of b-D- (l 4)-linked glucopyranosyl units, and that a side chain consisting of single D-xylopyranosyl unit attached to every 2nd ,3rd and 4th D-glucopyrnosyl unit through an a-D- (1,6) linkage. One D-galatopyranosyl unit is attached to one of the xylopyranosyl units through a b-D- (1,2) linkage.16 ,17
Nicotinic acid (NA) which is better known as niacin or vitamin B3, is so good at normalizing blood lipid levels that it has been declared the treatment of choice for reducing cholesterol by Expert Panel of the National Cholesterol Education Program (NCEP).18 The National Pharmacy Cardiovascular Council (NPCC) recognizes that NA favorable effects on the overall lipid profile make it a valuable treatment option for patients with atherogenic or mixed dyslipidemia, a condition characterized by elevated LDL-C and triglycerides.19
Despite its salutary effects NA has its own side effects also. Historically, the use of NA has been limited by cutaneous flushing, which is a prostaglandin D2-mediated vasodilation characterized by sudden warmth, redness, itching, and/or tingling on the face and truncal regions and is usually evident one to two hours after taking a dose of NA. Flushing can be reduced by giving aspirin and taking the medication with meals. 20
The present study is aimed to prepare the controlled release matrix tablets (CRMTs) of NA, which tends to reduce, but not eliminate, the notorious but harmless “flushing” effect caused by NA.
The present study is also aimed to evaluate the feasibility of using Xyloglucon XGL as hydrophilic matrix tablet polymer for controlling release of NA. A comparetative study with other hydrophilic polymers like Guar gum and Xanthan gum was also undertaken to understand the factors affecting drug release from these hydrophilic matrix tablets.
Materials and Methods
Materials
USP grade Nicotinic acid NA was obtained as a gift sample from Jagdale Scientific Research Foundation Bangalore. India. The USP grade of Xanthan gum and Guar gum was purchased from Vasa Chemicals, Bangalore and all other Chemicals provided by the P.G.Department of pharmaceutics, Dr.H.L.T.College of Pharmacy. Bangalore (Rural). India.
Methods
Isolation of Xyloglucan (XGL)
The isolation of XGL was performed by following the method reported earlier. 9,12 The 20 g of tamarind kernel powder was added to 200 ml of cold distilled water to prepare slurry. The slurry was poured into 800 ml of boiling distilled water. The solution was boiled for 20 min under stirring condition in a water bath. The resulting thin clear solution was kept overnight, and then the solution was centrifuged at 5000 rpm for 20 min. The supernatant liquid was separated and poured into twice the volume of absolute ethanol by continuous stirring to precipitate the polysaccharide. The precipitate was washed with 200 ml absolute ethanol and then dried at 50-60° for 10 hrs. The dried material was ground and sieved to obtain granules of different particle size range. The particle size range of 150-175 microns was used for preparation of tablets and stored in desiccator until further use.
Standard calibration curve of NA
Stock solution (SS) 100 mg /ml of NA was prepared by dissolving 10mg of NA in 10 ml of aqueous ethanol (95% distilled). A series of dilutions of SS were made to obtain a range of 2.5 mg to 20 mg /ml, and absorbance was noted by UV Spectrophotometrically at 216 nm. The standard calibration curve of NA is shown in Fig 1.
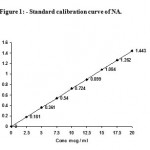 |
Figure 1: Standard calibration curve of NA.
|
Preparation of CRMT,s
The CRMTs of NA were prepared by Wet Granulation Method. First sieve all the ingredients with a # 60 sieve, weigh and collect the required quantity of sieved ingredients (Table 1), (except Lubricants.) and weigh the clinically relevant dosage strength of 300 mg NA. Different concentration of XGL, Xanthan gum and Guar gum, with NA (Table 1), were placed in a tray and mixed with sufficient quantity of granulating agent (Ethanol with respective gum) to make a coherent mass. This mass was immediately passed through a # 16 sieve, which was superimposed on # 44 sieve, then spread out on a tray, and dried in an oven at 500 for 1 hr. The dried granules were lubricated thoroughly with the weighed and sieved lubricants (Table 1) finally these lubricated granules were compressed into tablets using rotary tablet press machine.
Table 1: Formulation of CRMTs.
| Name of the Ingredients | Quantity / Tablet (mg) | ||||
| Formulation codes | |||||
| F1 | F2 | F3 | F4 | F5 | |
| Nicotinic acid | 300 | 300 | 300 | 300 | 300 |
| Xyloglucon | 50 | 100 | 150 | — | — |
| Xanthan gum | — | — | — | 100 | — |
| Guar gum | — | — | — | — | 100 |
| Lactose | 130 | 80 | 30 | 80 | 80 |
| Magnesium stearate | 06 | 06 | 06 | 06 | 06 |
| Talc | 14 | 14 | 14 | 14 | 14 |
| Total weight (mg) | 500 | 500 | 500 | 500 | 500 |
Standard physical test of CRMTs
All prepared CRMTs were evaluated for uniformity of weight and drug content. Hardness was measured by using Monsanto Hardness Tester. Friability of the tablets was determined by using Roche Friabilator.
Drug content Determination
20 tablets were weighed and powdered. Powder equivalent to 300mg of NA was shaken with 200 ml of aqueous ethanol (95% distilled). Transfer 1.0 ml of this solution to 100-ml volumetric flask, dilute with ethanol to volume, and mix. Concomitantly determine the absorbance of this solution and a solution of NA in the same medium, at a concentration of about 20 mg/ml, in 1-cm cells at a wavelength of maximum absorbance at about 262 nm, with UV Spectrophotometer.
In vitro release studies
In vitro release studies were carried out using tablets dissolution test Apparatus 11(Basket Method). The dissolution medium consisted of 0.1 N hydrochloric acid (pH 1.2) for first 2 hrs and phosphate buffer (pH 6.8) for subsequent 10 hrs. A quantity of 900 ml of the dissolution fluid was maintained at 37 ± 1° with a stirring speed of 100 ± 2 rpm used for the study. Aliquots of 5 ml were withdrawn at predetermined time intervals and an equivalent amount of fresh buffer maintained in the same temperature was replaced. Then absorbance was measured at about 262 nm with UV Spectrophotometer. The data presented were the mean of three determinations.
Swelling Behavior of CRMTs
The extent of swelling was measured in terms of percent weight gain by the tablet. The swelling behavior of formulation F2, F3, and F5, was studied. One tablet from each formulation was kept in petridish containing phosphate buffer (pH 6.8). At the end of 1hr tablet was withdrawn, soaked with tissue paper and weighed. The process is continued for 12hr. Percent weight gain by the tablets was calculated using formula.
S.I. = {(M t – M 0) / M 0} x 100
Where S.I. = swelling index, M t = weight of tablet at time ‘t’ and M 0 = weight of tablet at time t = 0.
Stability Studies
Stability studies was carried out to observe the effects of temperature and relative humidity (RH) on selected formulations F2, F3, and F5 by keeping them at room temperature ( 290, RH 60 ± 2 %), and at 450, RH 70 ± 1% in air tight high density polyethylene bottles for three months. The tablets were observed after every alternate week for changes in their physical characteristics
Results and Discussion
The present investigation was undertaken to formulate and evaluate the CRMTs of NA. All the tablet formulations were subject to various evaluation parameters and the results this obtained were found to be within the range (table 2). The weight variation test indicates that all the tablets were uniform with low standard deviation values. The hardness of all the tablets was in the range of 5.1 ± 0.2 to 5.3 ± 0.2 kg/cm2. The loss in total weight in friability test was in the range of 0.22 to 0.27 %. The percentage drug content for different formulations was varied from 99.68 ± 0.6 to 101.23 ± 0.9 % as shown in (table 2).
Table 2: Evaluation of CRMTs.
| Parameters | Formulation codes | ||||
| F1 | F2 | F3 | F4 | F5 | |
| Weight Variation (mg) * | 501
± 0.022 |
500
± 0.0877 |
501
± 0.026 |
503
± 0.023 |
502
± 0.017 |
| Hardness (Kg / cm2 ) * | 6.0
± 0.071 |
6.3
± 0.109 |
6.5
± 0.066 |
6.1
± 0.009 |
6.3
± 0.236 |
| Friability (%)* | 0.27
± 0.032 |
0.22
± 0.002 |
0.22
± 0.056 |
0.25
± 0.00 |
0.23
± 0.024 |
| Drug Content (%) * | 99.98
± 0.6 |
98.87
± 0.3 |
100.02
± 0.0 |
101.23
± 0.9 |
99.68
± 0.030 |
*Mean ± SD, n =3(All values are the average of three Determination)
The in vitro release was performed for 2 hrs in acidic buffer (pH 1.2) and followed by 10 hrs in phosphate buffer (pH 6.8). Drug release from the tablets containing xanthan gum F4 was found to be slightly faster in acidic media due to more rapid initial surface erosion than at higher pH compared to release from XGL and guar gum formulations. From the Fig-2 it is observed that all the formulations F1, F2, F3, F4 and F5 showed controlled but complete drug release in phosphate buffer (pH 6.8).
 |
Figure 2: – Dissolution profile of selected Formulations.
|
The swelling index was calculated with respect to time as given in Fig-3. As time increases, the swelling index also increased, because weight gain by tablets was increased proportionally with rate of hydration up to 3 to 4 hrs. Later on it decreases gradually due to dissolution of outermost-gelled layer of tablets into dissolution medium. The direct relationship was observed between swelling index and gum concentration. A gum concentration increases, swelling index also increased. It has observed that the cumulative percent drug release decreases with increase in concentration of gum and swelling index. The reason attributed to this fact is slow erosion of the gelled layer from the tablets containing higher amount of gum. Among all the formulations the swelling index of F3 was significantly more compared to F2 and F5 formulations. Graph was almost linear which suggests that the drug release was more by diffusion and less by erosion. Inverse relationship was noted between amount of gum and release rate of NA. Increase in the amount of gum in the formulation from 50%w/w to 150%w/w, resulted in slower rate, and decreased the amount of NA released from the tablets. Comparison between XGL, xanthan gum and guargum based formulation shows release of drug from formulation F3 was more slow when compared to formulation F2 and F5. This slow release is because of the formation of more thick gel like structure around the matrix, that controls the release of NA from matrix tablets. It can be concluded that, apart from guar gum, and xanthan gum the XGL can be successfully used as an effective matrix material to retard the release of NA for extended period of time.
 |
Figure 3: Swelling index behavior study of selected Formulation.
|
Acknowledgements
The Authors are grateful to the Chairman and the Principal of Dr.H.L.T.College of Pharmacy Bangalore (Rural), for providing all necessary facilities for the project and constant encouragement and financial support.
References
- Colombo P. Bettini R. Massimo G. Catellani P.L. Santi P. and Peppas N.A., Drug diffusion front movement is important in drug release control from swellable matrix tablets., J Pharm Sci., 84(8), 991-997. (1995).
- Vazquez M.J. Perez-Marcos B. Gomez-Amoza J.L. Martines. Pacheco R. Souto C. and Concheiro A., Influence of technological variables on release of drugs from hydrophilic matrices., Drug Dev Ind Pharm., 18,1355-1375.(1992).
- Talukdar M.M. Plaizier-Vercammen j., Evaluation of xanthan gum as a hydrophilic matrix for controlled release dosage form preparations., Drug Dev Ind Pharm., 19:1037-46. (1993).
- Bonferoni M.C. Rossi S. Tamayo M. Pedraz J.L. Dominguez Gil.A. Caramella., On the employment of I-Carrageenan in a matrix system 1. Sensitivity to dissolution medium and comparison with Sod carboxymethyl cellulose and Xanthan gum. J Control Release., 26:119-27. (1993).
- Ford J.L. Ribinstein M.H. McCaul F. Hogan J.E. Edgar P., lmportance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropyl methylcellulose matrix tablets., Int J Pharm., 40: 223-34. (1987).
- Risk S. Duru D. Gaudy D. Jacob M., Natural polymer hydrophilic matrix: influencing drug release factors., Drug Dev Ind Pharm., 20:2563-74. (1994).
- Sujja-areevath J. Munday D.L. Cox P.J. Khan K.A., Release characteristics of diclofenac sodium from encapsulated natural gum mini-matrix formulations., Int J Pharm., 139:53-62. (1996).
- Khullar P. Khar R.K. Agarwal S.P., Evaluation of guar gum in the preparation of sustained-release matrix tablets. Drug Dev Ind Pharm., 24: 1095-9. (1998).
- Dhopeshwarkar V. and Zatz J.L., Evaluation of xanthan gum in the preparation of sustained release matrix tablets. Drug Dev. Ind. Pharm., 19(9), 999-1017. (1993).
- KrishnaiahY.S.R. Karthikeyan R.S. Satynarayna V., Three layer guar gum matrix tablet for oral controlled delivery of highly soluble metoprolol tartarate. Int J Pharm., 241 (2), 353-366. (2002).
- Rao P.S. Ghosh T.P. Krishna S., Extraction and purification of tamarind seed polysaccharide., J Sci Ind Res., 4: 705. (1946).
- Sano M. Miyata E. Tamano S. Hagiwara A. Shirai T., Lack of carcinogenicity of tamarind seed polysaccharide in B6C3F mice., Food Chem Toxicol., 34:463-7. (1996).
- Burgalassi S. Panichi L. Saettone M.F Jacobsen J. Rassing M.R., Development and in vitro/ in vivo testing of mucoadhesive buccal patches releasing benzydamine and lidocaine. Int J Pharm., 133: 1-7. (1996).
- Rao P.S. Srivastava H.C., Tamarind in industrial gums. In: Whistler RL. Editor. 2nd Ed, New York: Academic Press; 369-411. (1973).
- Meier H. Reid J.S., Reserve polysaccharides other than starch in higher plants in Encyclopedia of plant physiology. NS: Plant Carbohydrates I: Intracellular carbohydrates. In: Loewus FA, Tanner W, editors. Vol. 134, Springer- Verlag; 418-71. (1982).
- Gerard T., Tamarind Gum In Handbook of water-soluble gums and resins. ill: Davidson RL, editor. USA: McGraw-Hili Book Co; 23.1-23.12, (1980).
- Gidley M.J. Lillford P.J. Rowlands D.W., Structural and solution properties of tamarind-seed polysaccharide., Carbohydrate Res., 214:299-3 14. (1991).
- The Expert Panel. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. JAMA., 269:3015-3023. (1993).
- McKenney J.M., Management of lipid disorders. In., McKenney J.M, Hawkins D., Edn. Handbook on the Management of Lipid Disorders. 2nd ed. St. Louis, MO: National Pharmacy Cardiovascular Council; 167-196. (2001).
- American Society of Health-System Pharmacists. ASHP therapeutic position statement on the safe use of niacin in the management of dyslipidemia. Am J Health Syst Pharm.; 54:2815-2819.(1997).
- Bari S.B and Kkashedikar S.G., Indian drugs scientific publication form., Indian drugs manufacturer association Vol .33., no.8. Page- 411. (August 1996).
- Roaymond M. In Lachman. Leon. Liberman H A. Joseph B. Schwartz., pharmaceutical Dosage Forms: Tablets Vol 1, 2nd Edn, by Marcel Dekker Inc.New York. 294,295,322,304. (1989).
- Rockville MD., The United States Pharmacopoeia 27 / National formulary 22, Asian Edn, United States Pharmacopeial convention, Inc., 1313 (2004).
- Banker G.S. Anderson N.R. In Lachman., Leon Liberman H.A. Knig J.L., Eds., The theory and Practice of industrial pharmacy, 3rd Edn, Varghese Publishing House, Mumbai., 297-300 (1987).
- Nath B.S. Venkatesh. and Hiremath., Formulation and evaluation of sustained release dosage form of a theophylline using a combined hydrophobic and hydorphyllic matrix., Indian J Pharm. Sci., 62(1), 33-36 (2000).
- Krajalic A. Jacker I.G., Matrix formation in sustained release tablets: possible mechanism of dose dumping. Int. Pharm.,251(1), 67-78 (2003).
- Crowley M.M. Schroedar B. Frederersdorf A. Talariko M. McGinity W., Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot melt extrusion. Int. Ph arm., 269(2)., 509-522 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.





