How to Cite | Publication History | PlumX Article Matrix
Hematological and Cytogenetic Responses to STI571 Therapy in Chronic Myeloid Leukemia
Amit K. Dutta1, N. Ganesh1 and N. K. Sah2
1Department of Research, Jawaharlal Nehru Cancer Hospital & Research Centre, Bhopal - 462 001 India.
2School of Biotechnology, Rajiv Gandhi Technical University, Bhopal India.
ABSTRACT: Chronic Myeloid Leukemia (CML), a malignancy/ cancer of the white blood cells (leukocytes), where in there is excessive multiplication of myeloid stem cells within the bone marrow, is caused by the Bcr- Abl tyrosine kinase, the product of the Philadelphia Chromosome. The hallmark of CML is the Philadelphia chromosome, which is present in more than 95% of patients. Imatinib mesylate, formerly known as STI-571 is a selective inhibitor of this kinase. A total of 25 patients had received imatinib mesylate therapy formed the subjects of this study. Patients were evaluated for cytogenetic and hematologic responses. Imatinib has shown remarkable clinical activity in patients with chronic myeloid leukemia. This study indicated that STI-571 has shown higher response rates compared with other therapy.
KEYWORDS: Chronic Myeloid Leukemia; Philadelphia; STI-571
Download this article as:| Copy the following to cite this article: Dutta A. K, Ganesh N,Sah N. K. Hematological and Cytogenetic Responses to STI571 Therapy in Chronic Myeloid Leukemia. Biosci Biotechnol Res Asia 2008;5(2). |
Introduction
Chronic Myeloid leukemia (CML) is a malignancy/ cancer of the white blood cells (Leukocytes), where in there is excessive multiplication of myeloid stem cells with in the bone marrow. Chronic Myeloid leukemia was the first human disease in which a specific abnormality of the karyotype – the Philadelphia chromosome could be linked to pathogenetic events of leukemogenesis. Philadelphia chromosome – the hallmark of Chronic Myeloid leukemia, is present in more than 95% of patients.1 This genetic abnormality generates a fusion oncogene, Bcr-Abl, which encodes a constitutively activated Bcr-Abl tyrosine kinase. In at least 90% of cases, this event is a reciprocal translocation termed t(9;22), which forms this particular chromosome.2,3
Chronic Myeloid leukemia is potentially curable with allogenic stem cell transplantation, but fewer than 30 percent of patients have suitably matched donors.3,4,5,6 Treatment with interferon alfa can induce a complete Cytogenetic response in 5 to 20 percent of patients and result in longer survival than that achievable with chemotherapy. Patients in whom interferon therapy fails are usually treated with hydroxyurea, busulfan, etc. The rate of hematologic response with these second line agents is approximately 50%, but cytogenetic responses are uncommon.
Imatinib mesylate (Gleevec, Novartis, Switzerland), a white to off-white to brownish or yellowish tinged crystalline powder, formerly called STI-571, is a potent and selective competitive inhibitor of the Bcr-Abl protein tyrosine kinase.7,8,9 Daily doses of 400mg or more of Imatinib induced hematologic responses in nearly all patients with chronic phase CML with minimal toxic effects. Activity was also Philadelphia chromosome – positive observed in some patients.
Materials and Methods
Peripheral Blood Sample, Heparin, RPMI-1640, PHA(Gibco), Colchicine(Gibco), KCl(Qualigens),
Carnoy’s Fixative, Trypsin, Saline(Merck), Giemsa(Qualigens), Gleevec(Novartis).
Laminar Air Flow (Rescholar), CO2 Incubator (Hear Cell),
Centrifuge (Remi), Cyclomixer,
Micropipette, Microscope (Olympus BX60).
Total twenty five cases were investigated and all the cases were already registered from Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal. A complete blood count and a differential blood count were obtained every fifteen days interval and cytogenetic analyses were evaluated every one-month interval. Heparinized peripheral blood samples were collected for cytogenetic analysis and transfer into 6 ml of RPMI-1640 media with the presence of mitogen. Cells were cultured, harvested and treated with KCl. Swollen cells were fixed, dropped onto a frosted slide and kept for ageing. Slide were then stained with giemsa (GTG banding performed and karyotyped) and observed under microscope (Olympus BX60).
Results and Observation
Hematological/ Clinical features of Patients
Table 1
| S.no | Details | STI571 | Others |
| 1 | Case studies | 20 | 5 |
| 2 | Age (yrs) | 47 | 48 |
| 3 | Sex (M/F) | 14 / 6 | 4 / 1 |
| 4 | Time from Diagnosis | 12 months | 12 months |
| 5 | Spleen Size (>10cm) | 14 | 5 |
| 6 | Hb (g/dl) | 12 | 11.8 |
| 7 | WBC (109/L) | 10 | 3 |
| 8 | TPC (109/L) | 12 | 4 |
Table 2
| Case No | Fever | Spleenomegaly | Hb (gm / %) | TLC (Cumm) | Total Metaphase Count | Ph Shows | Others Abnormality |
A1 |
– | + | 11.4 | 7100 | 13 | 04 | + |
| A2 | – | + | 12.7 | 5700 | 34 | 13 | – |
| A3 | + | + | 11.0 | 14900 | 27 | 09 | – |
| A4 | – | + | 10.7 | 5400 | 30 | 03 | – |
| A5 | + | + | 9.8 | 14700 | 45 | 12 | – |
| A6 | + | + | 13.2 | 9600 | 35 | 12 | – |
| A7 | + | + | 11.7 | 27000 | 44 | 14 | – |
| A8 | + | + | 11.6 | 8400 | 17 | 11 | – |
| A9 | – | – | 8.0 | 4800 | 13 | 09 | – |
| A10 | + | + | 10.5 | 6800 | 27 | 19 | – |
| A11 | + | – | 6.0 | 245000 | 09 | 05 | – |
| A12 | + | + | 13.4 | 18200 | 24 | 16 | – |
| A13 | + | + | 12.4 | 7500 | 20 | 11 | – |
| A14 | + | + | 10.3 | 10700 | 29 | 14 | – |
| A15 | + | + | 12.4 | 10500 | 30 | 10 | + |
| A16 | – | + | 11.0 | 55000 | 23 | 09 | – |
| A17 | + | + | 11.3 | 56000 | 34 | 07 | – |
| A18 | + | + | 10.8 | 13000 | 18 | 09 | – |
| A19 | – | + | 8.2 | 4000 | 34 | 10 | – |
| A20 | + | + | 12.4 | 8600 | 16 | 05 | – |
| A21 | + | + | 10.8 | 4500 | 34 | 05 | – |
| A22 | + | + | 12.8 | 6600 | 23 | 12 | – |
| A23 | – | – | 9.8 | 16000 | 19 | 06 | – |
| A24 | + | + | 12.3 | 5400 | 36 | 09 | – |
| A25 | + | |+ | 13.1 | 4900 | 23 | 02 | – |
Cytogenetic Observation
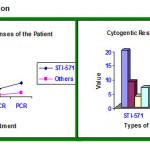 |
Graph 1
|
Table 3
| Sl. no | Response | STI-571 | Others |
| 1 | Total Case Studies | 20 | 5 |
| 2 | Major Cytogenetic(MCR) | 9 | 2 |
| 3 | Com. Cytogenetic(CCR) | 4 | 1 |
| 4 | Partial Cytogenetic(PCR) | 7 | 2 |
Photographs
 |
Figure 1
|
 |
Figure 2
|
 |
Figure 3
|
 |
Figure 4
|
Discussion and Conclusion
In the present study twenty-five cases (M: F = 21: 4) of chronic myeloid leukemia were studied. Their mean age was 32.8 (Range 06 years – 78 years). Out of these twelve had a rural background and thirteen had an urban background. Details of their age and sex distribution, clinical features and laboratory investigations are shown in the table.
All the 25 cases of chronic myeloid leukemia included in this study were Philadelphia chromosome positive. Philadelphia chromosome was first described by Nowell and Hungerford (1960). It has also been reported by Tough et al (1961), Fitzerald et al (1963) and Whang et al (1963), that during remission induced by therapy when the Philadelphia positive cells in peripheral blood cultures are almost depleted, the percentage of abnormal cell line with Philadelphia positive chromosomes in bone marrow is hardly affected. Only two cases of CML had abnormal karyotypes i.e. in the form of Polyploidy and Fragments apart of the sole abnormality t (9; 22) (q34; q11).
Our results are similar with Kemp et al. (1964), who reported hyperdiploidy unrelated to Philadelphia chromosome but was found with the onset of the disease. STI-571 is a rationally designed, potent and selective inhibitor of the Bcr-Abl tyrosine kinase that has shown efficacy in all three phases of chronic myeloid leukemia. Imatinib induced better hematological and cytogenetic response than other therapy.
Since our study has been a small one, it is not possible to draw a clear- cut conclusion for which a larger data based study is required. Further it is felt that an attempt should be made to extend such studies to various family members of patients.
References
- Savage DG, Antmann KH. Imatinib mesylate – a new oral targeted therapy. N Engl J Med. 2002; 346: 683 – 693.
- Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature.1973; 243:290 – 3.
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science. 1960; 132; 1497.
- Sawyers CL, Chronic myeloid leukemia. N Engl J Med. 1999; 340: 1330-40.
- Kantarjian HM, Deisseroth A, Kurzrock R, Estrov Z, Talpaz M. Chronic myelogenous leukemia: a concise update. Blood. 1993; 82: 691-703.
- Silver RT, Woolf SH, Hehlmann R, et al. An evidence based analysis of the effect of busulfan, hydroxyurea, interferon, and allogenic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood. 1999; 94: 1517- 36.
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Med. 1996; 2: 561-6.
- Deininger MW, Goldman JM, Lydon N, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of Bcr-Abl- positive cells. Blood. 1997; 90 : 3691-8.
- Druker BJ, Lydon Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest. 2000; 105: 3-7.
- Tough, IM; Court Brown WM; Baikie, AG; Buckton, KS; Harden, DG; Jawb, PA; King, MJ; McBridge, JA.(1961): Cytogenetic studies in CML and acute leukemia associated with mongolism. Lancet 1: p.411.
- Fitzgerald, PH; Crossen, PE; Hamer, JW. (1973): Abnormal Karyotypic clones in human acute leukemia and their nature and clinical significance. Cancer .1: p.1069.
- Whang, J; Frel, E; Tijo, JH; Carbone, PP; Brecher, G (1963): The distribution of Ph chromosome in patients with CML. 22: p.664.
- Kemp, NH; Stafford, JL. And Tanner, R (1964): Chromosome studies during early and terminal CML. British Medical Journal. 1: p.1010.

This work is licensed under a Creative Commons Attribution 4.0 International License.





