How to Cite | Publication History | PlumX Article Matrix
In vitro callus induction of Rosa hybrida L. Cv. Babylon
D. Teepica Priya Darsini1* and J. Anitha2
1Department of Biotechnology,
2Department of Bioinformatics, Karpagam University, Coimbatore - 641 021 India.
Corresponding Author E-mail: teepica@yahoo.com
ABSTRACT: In vitro study was carried out in the Rosa hybrida L.cv. Babylon to standardize the hormonal concentration for the callus induction using leaf bits as explant on Murashige and Skoog (MS) medium supplemented with different concentrations of auxins and cytokinins. MS medium with supplemented 0.5mg/L 2,4-D stimulated the callus intiation and the combination of 1.5mg/L 2,4-D and 0.1mg/L BAP concentrations was found to be suitable for maximum callus proliferation.
KEYWORDS: Callus; Leaf Bits; Rosa hybrida; Totipotency; Indirect organogenesis; Auxins; Cytokinins;
Download this article as:| Copy the following to cite this article: Darsini D.T.P and Anitha J. In vitro callus induction of Rosa hybrida L. Cv. Babylon. Biosci Biotechnol Res Asia 2008;5(2). |
| Copy the following to cite this URL: Darsini D.T.P and Anitha J. In vitro callus induction of Rosa hybrida L. Cv. Babylon. Biosci Biotechnol Res Asia 2008;5(2).Available from: |
Introduction
Roses have been for centuries, one of the most popular of ornamental plant. In terms of commercial flowering, roses are one of the top three in the world trade cut flowers. In India, roses are being grown for a long time and today are considered the principal cut-flowers crop grown in approximately 20% of the total area under ornamentals. Roses are propagated through direct and indirect organogenesis which enables substantial and fast multiplication, superior plant can be multiplied all the year round in large numbers and commercially exploited on large scale. This study was conducted to standardize the protocols for callus induction and somatic embryogenesis to further enhance the commercial value. (Lloyd, et al., 1988) (Burger, et al., 1988) reported that in vitro adventitious shoot differentiation in callus culture originated from leaf and stem bits and zygotic embryo segments of roses. Standardization of various auxins and cytokinins concentrations for callus initation in rose using leaf bits as explants in the cultivar Rosa hybrida L.cv. Babylon were tried out .
Materials and Methods
Rose plants were procured from Regional Agricultural research station, kerala Agricultural University, Ambalavayal and maintained in the pots. Young Leaves were collected from the portion of shoots and were used as explants. They were washed with liquid soap followed by running water wash for 10 minutes. Then the plant material was subjected with 70% ethanol for about 30 seconds, and then treated with 0.1% mercuric chloride treatment for about 2- 3 minutes, followed by several washes with sterile distilled water to remove the traces of the surface sterilants. The explants were inoculated into MS medium (Murashige and Skoog 1962) with different concentrations of 2,4-D as plant growth regulator, ranging from 0.1 – 0.9 mg /L for callus intiation. For the rapid callus proliferation, the initiated callus was inoculated into the medium supplemented with the varying concentrations of 2,4-D (1.0 -3.0 mg/L) and BAP (0.1- 0.2 mg/L) respectively. Sucrose as carbon source and agar (phytoagar) as jelling agent. pH of the medium was maintained to be 5.8. The photo period maintained was 18/6 hours dark /light at 1.0 ± 21°C for the callus initation and for rapid callus proliferation.
Results and Discussion
The hormonal concentration of 0.5mg/L 2,4-D was found to the optimum hormone concentration for the callus initiation among the various concentrations tried out. Maximum percentage of callus initiation was achieved in this hormone concentration and the number of days was found to be 14.7 days and the percentage of initiation 83% respectively (Table-1). Low concentrations of 2,4-D resulted in delayed and decrease in percentage of callus initiation and even high concentration above the optimum level of the hormone resulted in considerable reduction in callus initiation percentage. (Chi-ni Hsia and Schuyler S. Korban 1996) observed that pre incubation of somatic tissue of the cut rose ‘care free beauty’ and miniature ‘Red sunblaze’ and ‘Babykati’ in 10,100 or 200 μM/ L 2,4-D induced rhizogenic callus.
Table 1: Various concentrations of the Auxins on callus initiation of Rosa hybrida L.cv. Babylon.
| S.No | Concentration of 2,4-D (mg/L) | No of days for callus intiation | Percentage of Callus initiation |
| 1 | 0.1 | 33.2
±1.07 |
45 |
| 2 | 0.2 | 25.7
±1.07 |
58 |
| 3 | 0.3 | 21.8
±0.9 |
65 |
| 4 | 0.4 | 18.9
±0.8 |
70 |
| 5 | 0.5 | 14.7
±0.48 |
83 |
| 6 | 0.6 | 15.75
±0.68 |
75 |
| 7 |
0.7 |
15.9
±0.72 |
72 |
| 8 | 0.8 | 16.1
±0.8 |
69 |
| 9 | 0.9 | 16.2
±0.85 |
65 |
The hormonal concentration of 1.5mg/L 2,4-D and 0.1mg/L BAP was found to the optimum hormone concentration for rapid callus proliferation or multiplication among the various concentrations tried out. Number or amount of callus, No of days for callus proliferation and Percentage for the following parameters were recorded to confirm the above statement, was recorded as 3.6, 18 and 91% respectively (Table-2). (Rout, Debata and P.Das 1991) reported that callus was initiated from immature leaf and stem segments of Rosa hydrida L. cv. Landora and sub cultured every four weeks on basal half MS medium supplemented with 2.2-9.9 μM/ L 2,4-D and 2.2 μM/L BAP and 5.4 μM/L NAA.
Table 2: Various Concentration of the Auxins & cytokinins for Callus muliplication of Rosa hybrida L.cv. Babylon.
| S. No | Concentration of 2,4-D and BAP (mg/L) | Number or amount callus Proliferation | No of days for callus Proliferation | Percentage of callus Proliferation |
|
1 |
1.0, 0.1 |
0.9 ± 0.08 |
20.7
±0.74 |
70
|
| 2 |
1.5,0.1 |
3.6
± 0.47 |
18
±0.57 |
91 |
| 3 |
2.0, 0.1 |
1.8
± 0.68 |
18.5
±0.95 |
80
|
| 4 |
2.5, 0.1
|
1.6 ± 0.74 |
18.7
±0.98 |
75
|
| 5 |
3.0, 0.2 |
1.85 ± 0.09 |
19.6
±0.94 |
68
|
Further, the tubes with of low concentration of 2,4-D below the optimum level resulted in decreased percentage of callus. However, with the increase in concentration of 2,4-D also resulted in decrease of the above-mentioned parameters. Therefore, it becomes necessary to confirm that, though the concentration 2,4-D was increased above 1.5mg/L in combination with 0.1 mg/ L BAP the rapid proliferation of callus considerably reduction , with the above mentioned parameters, with the increasing concentration of 2,4-D and BAP. It has to be concluded that increased and decreased concentration of phytohormones above the optimum level inhibited the callus formation considerably.
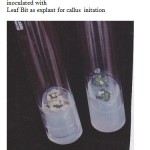 |
Figure 1: MS medium with supplemented 0.5mg/L 2,4-D inoculated with Leaf Bit as explant for callus initation.
|
 |
Figure 2: MS medium with supplemented 1.5mg/L 2,4-D and 0.1mg/L BAP for rapid callus proliferation.
|
Conclusion
MS media can be supplemented other chemical components for induction of somatic embryogenesis. Auxin and cytokinin ratio has to be standardized for regeneration the callus into entire plantlet.
References
- Burger,D., Liu, W.L. Zary, K.W. and Lee, C. I .“Organogenesis and plant regeneration from immature embryos of Rosa L. hydrida .” Plant Cell Tissue organ Culture, 21: 147-152. (1990).
- Chi-ni Hsia and Schuyler S. Korban . “Organogenesis and Somatic Embryogenesis in callus cultures of Rosa hybrida and Rosa Chinensis Minima .” Plant Cell, Tissue Organ Culture, 44 (1) : 1-6. (1996).
- Lloyd, D., Roberts, A.V. and Short, K.C. “The Induction of in vitro adventitious shoots in Rosa”. Euphytica. 37: 31-36. (1988).
- Murashige, T. and Skoog, F. “A revised medium for rapid growth and bioassays with tobacco cultures.” Physiol. Plant., 15 : 473 97. (1962).
- Rout, G.R. Debata, B.K. and Das, P. “Somatic embryogenesis in callus cultures of Rosa hydrida L. cv. Landora”. Plant Cell, Tissue Organ Culture. 27:65-69. (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





