How to Cite | Publication History | PlumX Article Matrix
Influence of Smoking on Activity of Salivary A-Amylase
R. Sariri*, R. H. Sajedi, H. Ghafoori and A. Varasteh
Department of Biochemistry, The University of Guilan, Rasht (Iran).
ABSTRACT: Tobacco smoke is involved in the pathogenesis of several diseases regarding different body systems. Saliva is the first body fluid to confront inhaled cigarette smoke which is injurious to the oral cavity and is associated with several oral diseases and cancer.The aim of our study was to evaluate the influence of smoking on the activity of a-amylase, in the saliva of healthy smoker individuals. a-amylase was measured in the supernatant of centrifuged saliva of 25 volunteers smokers, before and just after smoking a single cigarette using the standard chemical methodsThe enzymatic activity showed a significant inhibition following a single cigarette. Reduction in the enzymatic activity of saliva is most probably due to the interaction between smoke aldehydes and –SH groups of the enzyme molecules Based on the results obtained in the present study, it could be emphasized that smoking of just one cigarette is sufficient to alter the salivary a- mylase enzymatic activities.
KEYWORDS: Salivery enzymes; a-amylase; cigarrete smoking
Download this article as:| Copy the following to cite this article: Sariri R, Sajedi R. H, Ghafoori H, Varasteh A. Influence of Smoking on Activity of Salivary A-Amylase. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Sariri R, Sajedi R. H, Ghafoori H, Varasteh A. Influence of Smoking on Activity of Salivary A-Amylase. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7199 |
Introduction
Tobacco smoke is involved in the pathogenesis of several diseases regarding different body systems, mainly cardiovascular and respiratory in addition to its local toxic effect in the oral cavity. The noxious effects of smoke compounds explains the reason for high incidence of periodontal diseases, caries, and neoplastic diseases of oral tissues in smokers. Cigarrett smoke is seriously injurious to the oral cavity and is associated with several oral diseases and cancer. It contains about 4000 different chemicals, 1/10th of which are known carcinogens. Tobacco smoke also contains potent oxidants such as oxygen free radicals and volatile aldehydes [1, 2]. The oxidizing agents can seriously damage biomolecules such as proteins and enzymes leading to various physiological problems.
Saliva is the first biological fluid to encounter the cigarette smoke. Saliva is well known for its highly protective functions against deterious agents such as microorganisms, toxines and various oxidants [3]. The antioxidant capacity and reducing power of saliva may diminish to a high degree due to various factors [4]. It has been shown that in vitro exposure to cigarette smoke could significantly decrease some enzymatic activities, both in plasma and in saliva [5]. There exist some toxic components of tobacco smoke, unsaturated and saturated aldehydes, that are able to interact with thiol rich compounds, leading to structural and functional modification of these molecules. It has been found that addition of glutathione (GSH) can cause a decrease in the damaging role of smoke aldehydes [6].
This study reports on the influence of habitual cigarette smoking on a-amylase activity in individuals yet to manifest any physical or clinical sign associated with such smoking habit.
Materials and Methods
A commercially available direct a-amylase kit based on the hydrolysis of a substrate by a-amylase in the presence of a chromogen was used (Chem Enzyme). a-Amylase activity was determined in supernatant of saliva samples collected from volunteers.
Volenteers
Twenty-four heavy smokers and equivalent number of similar sex, age and weight-matched non-smokers, all in apparent good health were enlisted for the study. A precise consent was obtained from each individual and a dentist examined their mouth and teeth for the presence of any oral cavity or teeth abnormality before each sample collection.
Cigarretes
The type of cigarrettes they used were polpular commercial cigarrettes containing 14 mg of tar and 0.9 mg of nicotine. Both group were in the age range of 20 to 28 years, and the smokers had the habit of smoking 5-15 cigarretes per day. The men volenteers came to the laboratory and provided saliva samples. Fifteen minutes after providing the saliva sample, the smoker group were asked to smoke their cigarrete in a way they were used to. Another sample of their saliva was collected within 60 minutes after smoking.
Saliva collection
The subjects were examined by a dentist for the presence of infection or other symptoms of oral/and or dental disorders. They were then asked to gaggle the mouth with about 5.0 ml of distilled water for about 2 minutes and thereafter, saliva samples (1 ml) were collected, without exogenous stimulation. All saliva samples were collected no less than 1.5 hours after the meal. The samples were immediately centrifuged (1500g, 15 min) at 4°C to remove squamous cells and cell derbies and stored frozen until assay. They were analyzed within 48 hours of collection. Sample assays were again performed after one month using fresh samples collected from the same set of volunteers and the data obtained were expressed as mean + SD of the two determinations.
a-Amylase assay
In this research salivary a-amylase was measured using 2-chloro-4-nitrophenyl-a-D-maltotrioside (CNPG3) as substrate, in which a chromogen 2-chloro-4-nitrophenyl is attached to a molecule of maltotrioside. This direct amylase assay does not need enzymes such as a-glucosidase/glucoamylase and, therefore, attracted much attentions by researchers.
CNPG3 is hydrolysed by a-amylase producing 2-chloro-4-nitropheny (CNP) directly and the concentration of CNP is measured at 405 nm. The reaction is fast enough without the need of using additional enzymes.
10 CNPG3 → 9 CNP + CNPG2 + 9 G3 + G
In a typical reaction at 37°C, 25 ml of the saliva sample was added to 1 ml of the substrate reagent and mixed rapidly. Absorption was measured at 405 nm after exactly one minute followed by a second measurement after 5 minutes. The increase in absorption was related to the activity of a-amylase.
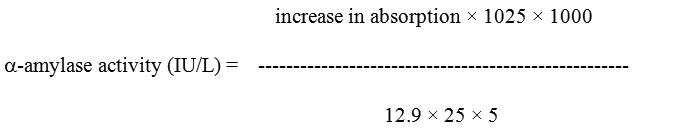
The absorption coefficient for 1 mM of CNP at 405 nm is 12.9 and 1025 and 25 are the total and sample volumes respectively.
Results
The activity of a-amylase in saliva of smokers and non-smokers is presented in Tables 1 and 2. The data in Table 2 were obtained after one month in a similar manner and a mean value of the two sets of data was used to obtain the respective graphs. Figure 1 shows the mean values of a-amylase activity in smokers and none smokers both at the beginning of the study and one month later. The values for non-smokers did not change significantly after one month, while in the case of smokers the activity of salivary a-amylase decreased by a factor of about 4%. As the figures in Table 1 and Figure 1 indicate, the activity of amylase is lower (about 60%) in heavy smokers compared to the non-smoker group. The figures also show that in the case of smokers, the enzyme activity is even lower following smoking of one cigarrete compared to the beginning of the study when the smoker subjects had not smoke for at least 3 hours. Electrophoresis of tear proteins in the case of smokers and non-smokers also (Figure 2) reflected the same results, i.e. reduction in the amount of many salivary proteins.
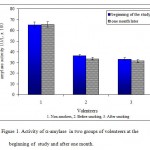 |
Figure 1: Activity of a-amylase in two groups of volenteers at the beginning of study and after one month.
|
Discussion
The enzymatic activity of a-amylase showed an inhibition in the smoker group, especially after smoking their cigarette. This is probably due to the interaction between smoke aldehydes and –SH groups of the enzyme molecules. Moreover, the percentage of the enzymatic inhibition showed a negative correlation with the basal level of salivary reduced gluthation (GSH) . Our results emphasize that not only one cigarette is sufficient to impair the salivary enzymatic activities but also strengthen the proposed protective role of GSH against the noxious biochemical effects of CS.
Salivar a-amylase is key for extracting caloric value from food. However, beyond the primary role of a-amylase to begin digestion of complex starches, sugars, and carbohydrates [7], salivatory a-amylase is known be a surrogate marker of physiobiology of stress [8]. It has also been shown that salivary a-amylase may be infuenced by behavioral and psychological factors and processes [9].
Conclusion
Normal salivary function is considered to be critical for the maintenance of healthy oral mucosa [10]. Oral fluids provide an easily available non-invasive for the diagnosis of a wide range of diseases and clinical situations. Determination of biological activity of a-amylase is a non-invasive method for various factors that may influence oral biochemistry [11]. The fact that salivary a-amylase is the key enzyme for extracting caloric value from foods, highlights the importance of studying its inhibitors in the saliva. Inhibition of the enzymatic activity of the enzyme due to aldehydes present in cigarrete smoke would directly influence the digestion process, especially in the case of carbohydrates. On the other hand, as the oral digestion of complex carbohydrates is more efficient under conditions of deep relaxation [8], smoking will interfere with body’s relaxtion state and, therefore, retards carbohydrate digestion. It has been shown that exposure to CS in-vitro has reduce the activity of amylase, lactate dehydrogenase (LDH) and acid phosphatase, but the it had very little effect on the activity of alkaline phosphatase and aminotransferases [5]. However, to our knowledge, this study was the first to demostrate the inhibitory effect of cigarrete smoking on the biological activity of a-amylase in-vivo. We are also investigating the effect of cigarrete smoke on salivary enzymes (both in-vitro and in-vivo) to show the influence of cigarrete type, exposure duration and the chemical composition and concentration of inhibitors on the inhibition process.
Table 1: The activity of a-amylase at the beginning of the study.
|
Volenteer |
a-amylase activity (IU/L) in saliva of: | ||
| Non-smokers | Smokers
(before smoking) |
Smokers
(1 hour after smoking) |
|
| 1
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 |
62 × 103
66 × 103 71 × 103 70 × 103 68 × 103 65 × 103 61 × 103 67 × 103 66 × 103 66 × 103 67 × 103 65 × 103 65 × 103 64 × 103 61 × 103 60 × 103 65 × 103 62 × 103 66 × 103 71 × 103 70 × 103 68 × 103 65 × 103 67 × 103 |
35 × 103
34 × 103 36 × 103 35 × 103 36 × 103 37 × 103 37 × 103 36 × 103 35 × 103 29 × 103 38 × 103 37 × 103 36 × 103 35 × 103 36 × 103 34× 103 36 × 103 35 × 103 29 × 103 38 × 103 37 × 103 36 × 103 35 × 103 34× 103 |
32 × 103
32 × 103 33 × 103 34 × 103 35 × 103 35 × 103 33 × 103 31 × 103 31 × 103 32 × 103 34 × 103 33 × 103 34 × 103 33 × 103 35 × 103 33 × 103 32 × 103 31 × 103 31 × 103 32 × 103 34 × 103 33 × 103 34 × 103 35 × 103 |
| Mean | 65.2 × 103 | 36.3 × 103 | 33.2 × 103 |
Table 2 The activity of a-amylase one month later.
|
Volenteer |
a-amylase activity (IU/L) in saliva of: | ||
| Non-smokers | Smokers
(before smoking) |
Smokers
(1 hour after smoking) |
|
| 1
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 |
62 × 103
65 × 103 71 × 103 71 × 103 69 × 103 63 × 103 61 × 103 67 × 103 66 × 103 66 × 103 67 × 103 65 × 103 65 × 103 64 × 103 61 × 103 60 × 103 66 × 103 61 × 103 67 × 103 71 × 103 71 × 103 65 × 103 65 × 103 67 × 103 |
32 × 103
33 × 103 33 × 103 34 × 103 36 × 103 36 × 103 36 × 103 35 × 103 33 × 103 28 × 103 35 × 103 35 × 103 33 × 103 33 × 103 33 × 103 32 × 103 34 × 103 34 × 103 29 × 103 35 × 103 36 × 103 35 × 103 34 × 103 32 × 103 |
31 × 103
32 × 103 32 × 103 33 × 103 35 × 103 33 × 103 32 × 103 30 × 103 31 × 103 30 × 103 32 × 103 30 × 103 32 × 103 33 × 103 31 × 103 30 × 103 32 × 103 31 × 103 30 × 103 30 × 103 32 × 103 33 × 103 33 × 103 32 × 103 |
| Mean | 65.3 × 103 | 33.5 × 103 | 31.2 × 103 |
Figures:
Figure 1. Activity of a-amylase in two groups of volenteers at the beginning of study and after one month.
References
- Pryor W.A., Prier D.G., Church D.F. Environ Health Prospect 1983; 47: 345-55
- O’Neill, C.A., Halliwell B., van der Vliet A., van der Vliet A., Davis P.A., Packer L., Tritschler H., Storhman W., Reiland T., Cross C.E. Reznick A.Z. J. Clin. Med 1994; 124: 359-70
- Tabak L.A., Levine M.J., Mandel I.D., Elison L.A. Oral Pathol 1982; 11: 1-7.
- Kohen R., Tirosh O., Kopolovich K. Gerontol. 1992; 27: 161-68.
- Nagler R., Lischnisky S., Diamond E., et al. Effect of cigarette smoke on salivary proteins and enzyme activities. Arch Biochem Biophys 2000; 379(2): 229-36.
- Zappacosta B., Persichilli S., Mordente A., et a Inhibition of salivary enzymes by cigarette smoke and protective role of glutathione. Human and Experimental Toxicology 2002; 21(1): 7-11.
- Lebanthal E. Role of salivary amylase in gasteric and intestinal digestion of starch. Digestion, Diseases, Sci. 1987; 32: 1155-57.
- Morse D.R., Schachterele G.R., Furst L., Zaydenberg M., Pollack R.L. Oral digestion of a complex carbohydrate cereal: effects of stress and relaxation on physiological and salivary measures. J. Clin. Nutrition 1989; 49: 97-105.
- Kivlighan K.T., Granger A. Salivary a-amylase response to competition: Relation to gender, previous experience and attitudes. Psychneuroendocrinology 2006; 31: 703-14.
- Mayes, P. A. Digestion and absorption. In: Harpers, Biochemistry, K. Murray, D. K. Granner, P. A. Mayes, and V. W. Rodwell, 25th (ed), New York: Appleton and Lange; 2000. p. 66-674.
- Rauscher, E., Neumann, E.S., Bulow, S. and Wahlefeld, A W. Enzymatic determination of a-amylase activity in biological sample, Chem. 1995; 31:14-19.

This work is licensed under a Creative Commons Attribution 4.0 International License.





