How to Cite | Publication History | PlumX Article Matrix
Interaction pattern of Tridax procumbans lectins with erythrocytes of normal and diabetic patients
A.P. Ramteke and M.B. Patil
University Department of Biochemistry, RTM Nagpur University, Nagpur - 440 033 India.
ABSTRACT: The results of interaction between lectins of Tridax procumbans L and erythrocytes from the normal and diabetic patients show that as the level of blood glucose increases the hemagglutination titre decreases.
KEYWORDS: Diabetes mellitus; Lectins; Tridax procumbans
Download this article as:| Copy the following to cite this article: Ramteke A. P, Patil M. B. Interaction pattern of Tridax procumbans lectins with erythrocytes of normal and diabetic patients. Biosci Biotechnol Res Asia 2008;5(2). |
Introduction
Most of the diseases are known to be associated with changes in the glycosylation profiles of membranes proteins. The glycosylation patterns reflect the internal and external environment of the cells. The glycosylated proteins act as sensitive indicators of alterations in cell function brought about by the disease. Changes in the glycosylation are therefore being widely used as markers both for initial diagnosis and for following the disease progress 1, 2.
Lectins or hemagglutinins have the ability to recognize and bind with specific carbohydrate residue on cell surface membrane. This specific property of these proteins has been widely employed in biochemical research 3, 4. Lectins bind to the specific terminal of carbohydrate residues of glycoconjugate on the cell surface and have therefore been used as specific agents to study cell surface
structure and structural component of cell membrane 5.
Plant lectins have the ability to recognize complex glycoconjugates present on the cell surface and can be used to detect the alteration in the erythrocytes by manifesting significant changes in the hemagglutination titre. Noticeable changes in hemagglutination patterns, glycosylation profiles of normal and diseased cells are reported by Goldstein and Hayes (1978) 5.
In the present paper the hemagglutination pattern of erythrocytes of insulin dependent and non – insulin dependent Diabetes mellitus patients has been compared with the hemagglutination pattern of erythrocytes of normal persons using the lectins of T. procumbans L.
Materials and Methods
Leaves, stem and calyx of the plant T. procumbans L (Family – Compositeae) were used as the source of lectin 6. Papain, bovine serum albumin, guar – gum, D – galactose were obtained from Sigma
Chemicals St. Louis Mo. USA. Other chemicals were of analytical grade.
Blood samples of normal persons and diabetic patients were collected in citrate bulbs from blood bank, and clinical biochemistry laboratory of local hospital, Nagpur.
Isolation of lectins
Leaves, stem and calyx of 45 days old plants were collected from the garden of University Department of Biochemistry, RTM Nagpur University Nagpur, washed four to five times under the tap water and twice with distilled water and soaked between the folds of filter paper and homogenized separately for extraction of lectins as described by Ramteke and Patil, (2005) 7. Lectins were purified to homogeneity by affinity chromatography by the method of Dixon, (1953), 8. The lectins were characterized as described earlier9.
Protein estimation
Protein estimation was carried out by the method of Lowry et al., (1951) using B. S. A. as standard protein10.
Agglutination assay
The method of Deshpande and Patil (2002) was used to perform agglutination assay, using 2% suspension of papain treated erythrocytes of normal and diabetic patients 9. The hemagglutination titre was determined by serial dilution in 96 U well plates. The reciprocal of the last dilution showing detectable agglutination was taken as the titre strength of the lectin and expressed as Hemagglutination Units (HAU) 11.
Evaluation
The hemagglutination activity of erythrocytes of normal persons and diabetic patients (NIDDM, IDDM) is compared to student’s t test. The results were analysed with suitable statistical analysis (Standard Deviation, Critical Difference, and Correlation Coefficient) by the method of Persons 1947, using the following formula 12.
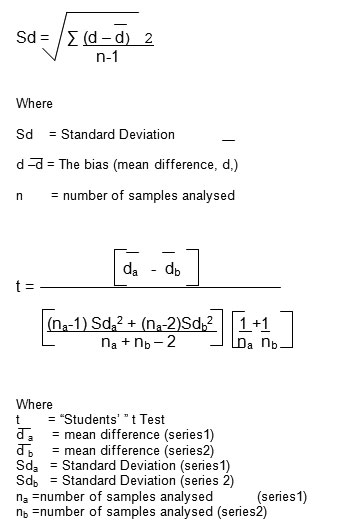
The difference in control group and diabetic cases were considered significant if p < 0.01 12, 13.
Results and Discussion
Tridax procumbans L was found to contain galactose specific lectin in leaves, stem and calyx. The lectins were purified to homogeneity by affinity chromatography and were found to have molecular weight of 23kD, 20kD, and 23kD respectively. Results presented in Table 1 and 2 show that there is a gradual significant reduction in the hemagglutination titre with the duration of diabetic condition in NIDDM patients, whereas erythrocytes of the IDDM patients show a significant drastic decline in the titre as compared to the erythrocytes of normal individuals. Changes in the hormones and insulin deficiency are known to effect macromolecular synthesis. Membrane proteins get glycosylated during diabetic conditions and undergo complex changes 14.
procumbans leaves stem and calyx lectins showed similar hemagglutination pattern with erythrocytes of insulin dependent and non – insulin dependent diabetic patients. Results illustrated in Table 1 and 2 – showed significant decrease in hemagglutination units with increase in the blood glucose. Nagada and Deshmukh (1998) reported similar results, with P. tithymaloid lectin. They also found a gradual decrease in hemagglutination units with the increase in the blood glucose level 13. Similar comparative study carried out by Marques et al., (2000) from normal and diabetic patients also reported that diabetic disorders appear to be associated with quantitative alterations of erythrocytes then the normal controls‑14. These results (Table 1 and 2) clearly demonstrate that there is an increase in diabetic condition and decrease in the hemagglutination titre as compared to the control. Thus the leaves stem and calyx lectins of T. procumbans L can be used in the medicinal chemistry research, as lectins are used extensively to investigate changes in the hemagglutination pattern, allowing new insights into the biological and pathological significance of glycosylation and may help in the diagnosis and treatment therapy in Diabetes mellitus15.
Table 1: Hemagglutination pattern of Tridax procumbans lectins with erythrocytes of normal persons and diabetic patients.
| Conditions | Number | Fasting Blood glucose | Hemagglutination Titre | CC | CD |
| and Duration | of cases | Average (mg/dl) | |||
| TPL – L TPL – S TPL – C | |||||
| Control | 86 | 98.09±5.67 | 16 – 64 8 – 32 16 – 64 | NA | NA |
| NIDDM | 177 | 217.3±21.68 | 4.96 | 0.18* | |
| 0 –2 years | 80 | 16 – 32 8 – 16 16 – 32 | |||
| 2 –10 years | 67 | 8 – 32 4 – 16 8 – 32 | |||
| 10 years and above | 30 | 8 – 16 4 – 8 8 – 16 | |||
| IDDM | 73 | 357.2±70.14 | 2.55 | 0.36** | |
| 0 –2 years | 30 | 4 – 8 2 – 4 4 – 8 | |||
| 2 –10 years | 35 | 2 – 8 1 – 4 2 – 8 | |||
| 10 years and above | 8 | 2 – 4 1 – 2 2 – 4 |
*p=0.002, **p=0.05, SD – Standard Deviation, CC – Correlation Coefficient, CD– Critical Difference, TPL – L – Tridax procumbans leaf lectin, TPL – S – Tridax procumbans stem lectin, TPL – C –Tridax procumbans calyx lectin, NIDDM – Non – Insulin dependent Diabetes mellitus, IDDM – Insulin dependent Diabetes mellitus.
Table 2: Hemagglutination Units of Tridax procumbans lectins with erythrocytes of normal persons and diabetic patients.
|
Conditios |
No of cases
|
Average titre
|
Hemagglutination units per ml
|
CC |
CD
|
| TPL – L TPL – S TPL – C | TPL – L TPL – S TPL – C | TPL – L TPL – S TPL – C | TPL – L TPL – S TPL – C | ||
| Control
NIDDM 0 – 2 years 2–10 years 10years and above |
86
177
80
67
30 |
37 ± 11.99 18.7 ± 9.89 37.3 ±11.99
28 ± 6.45 14 ± 3.48 28 ± 6.45
21.1 ± 7.93 8.83 ± 3.62 21.1 ± 7.93
13.4 ± 3.84 6.67 ± 1.92 13.4 ± 3.84 |
2560 1280 2560
1280 640 1280
1280 640 1280
640 320 640 |
NA NA NA
0.39 0.47 0.39
0.73 0.53 0.73
0.69 0.69 0.69 |
NA NA NA
3.9**** 6.12** 3.9****
5.10** 5.2**** 5.10**
4.69* 3.59* 4.69* |
| IDDM
0 – 2 years 2–10 years 10years and above
|
73
30 35
8 |
5.73 ± 2.02 2.87 ± 1.01 5.73 ± 2.02 5.65 ± 2.41 2.98 ± 1.06 5.65 ± 2.41 3.5 ± 0.33 1.75 ± 0.52 3.5 ± 0.33 |
1280 640 1280
320 160 320
160 320 160 |
0.16 0.16 0.16
0.15 0.19 0.15
0.45 0.45 0.45 |
6.39*** 4.86**** 6.39***
7.49**** 5.52** 7.49****
1.85NS 1.34NS 1.85NS |
Acknowledgement
Authors express sincere thank to the Head, Department of Biochemistry, and staff, Blood Bank, Indira Gandhi Medical College and Hospital, Nagpur for their kind Co-operation and grateful to Head, University Department of Biochemistry, RTM Nagpur University, Nagpur for encouragement.
References
- Hsu, S.M. and Raine, L. Versatility of biotin-labeled lectins and avidin-biotin-peroxidase complex for localization in tissue sections. J. of Histochem and Cytochem; 30, 157-161(1982)
- Zabel, P.L., Noujaim, A. A., Shysh A. and Bray J. Radio iodinated peanut lectin: a potential radio pharmaceutical for immunodetection of carcinoma expressing the T antigen. Eur. J. of Nuclear Medicine; 8, 250-254, (1983)
- Lis, H., and Sharon N., The biochemistry of plant lectins. Annu. Rev. Biochem; 42, 541-578, (1973)
- Goldstein, I. J., Hayes C. E., The Lectins; Carbohydrate – binding proteins of plants and animals. Adv. Carb. Chem. Biochem; 35, 127-340, (1978)
- Sharon, N. and Lis, H., Lectins as cell recognition molecules. Science; 246, 227-234, (1989)
- Ugemuge, N. R., Flora of Nagpur District, First Edition, Shri Prakashan, Nagpur, p216.
- Ramteke, A. P., and Patil, M. B., (2005) Purification and characterization of Tridax procumbans stem lectin. Biotech. Biosci. Res. Asia; 3(1), 103 – 110, (1986)
- Dixon, M., Nomogram for ammonium sulphate solution. J. Biochem; 54, 457-458, (1953)
- . Deshpande, K., and Patil, M., Studies on lectins of medicinal plants. Ph. D. Thesis, Nagpur University, Nagpur, (2002)
- Lowry, O. H., Rosebrough, N, J., Farr, A. L., and Randall, R, J., Protein measurement with the Folin phenol reagent. J. Biol. Chem; 193, 265-275, (1951)
- Deshpande, K., and Patil, M., Ind. Vet. Med. J; 25, 385-387, (2001)
- Sir Austin Bradford Hill, (Tenth Edition) A short textbook of medical statistics The English Language Book Society, Hodder and Stoughton London p.86, 173, 285, 297, 313
- Nagada, K., and Deshmukh, B., Hemagglutination pattern of galactose specific lectin from Pedilanthus tithymaloids in Diabetes mellitus. Indian J. Expt. Biol. 36, 426-428, (1998).
- Marques, F., Crespo, M. E., Silva, Z. I., Bicho, M., Insulin and high glucose modulation of phosphatase and reductase enzymes in the human erythrocytes: a comparative analysis in normal and diabetic patients. Diabetes Res. Clin. Pract., 47(3), 191-198, (2000).
- Mukherjee, N., Biswas, T. K., Mitra, S., Gupta, N. and Sarkar, M. Immunochemical correlation in Diabetes mellitus, a preliminary report on the lectin-agglutinin test. Association of Physicians of India; 39, 172-174, (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





