How to Cite | Publication History | PlumX Article Matrix
Purification and Identification of 70 Kda Heat Stable Protein
Aara Rifat, S. A. Hajam and K.I. Andrabi
Department of Biochemistry, University of Kashmir, Kashmir - 190 006 India.
ABSTRACT: A heat stable protein (hsp) has been purified to homogeneity from sheep liver extract by heating to 95 O C followed by ion-exchange chromatography on DEAE-cellulose (DE-52). The purified protein showed a single band when examined by gel electrophoresis. The molecular weight determined by SDSpage was found to be approximately 70KDa. The presence of this protein was also demonstrated in whole cell homogenate, heat stable and nuclear fraction.
KEYWORDS: Hsp; SDS-page
Download this article as:| Copy the following to cite this article: Rifat A, Hajam S. A, Andrabi K.I. Purification and Identification of 70 Kda Heat Stable Protein. Biosci Biotechnol Res Asia 2008;5(2). |
| Copy the following to cite this URL: Rifat A, Hajam S. A, Andrabi K.I. Purification and Identification of 70 Kda Heat Stable Protein. Biosci Biotechnol Res Asia 2008;5(2). Available from: |
Introduction
Many of the stress induced heat shock proteins such as Hsp90, Hsp70 and the small heat shock proteins are isoforms of the constitutive chaperones and perform similar or identical functions. Within the cell cycle processes and signal cascades, chaperones play important stabilizing roles. They associate with cell cycle or signal proteins in order to translocate them to their targets to keep them in required conformational state or to eliminate degraded or mutated forms.The constitutive members of this family in the cytosol are particularly involved in folding and refolding of proteins, in maintaining their unfolded (transport) state and in the elimination of non-functional proteins (Chirico et al 1988, Deshaies et al 1988, Cheng et al 1989, Beck man et al 1990). They do this by cooperating with so called co-chaperones. This view allows a unification of Hsp70 family function in which Hsc70 recognizes such transiently abnormal proteins in normal cell and in stressed cells, Hsc function is augmented by Hsp70 to handle proteins damaged directly or indirectly by stress (Skowyara et al 1990). However in stressed cells, the association is long lasting and could deplete Hsc 70 pools creating a deficiency in Hsc70 functions. Hsc70 also hands off unfolded polypeptides to mitochondrial Hsp70 (mt Hsp70) via the membrane translocation system. Mitochondrial Hsp 70 in turn may pass off at least some of these polypeptides to Hsp60/ Cpn10 for folding.
Methodology
Preparation Of Crude Liver Extract
Frozen sheep liver was cut into small pieces then homogenized in lysis buffer containing (10mM Tris acetate pH=7.5, 10mM NaCl, 1mMEDTA, 1mMPMSF) using a hand held homogenizer. The homogenate was centrifuged at 700g for 30 minutes Pellet was discarded and the supernatant recentrifuged at 700g for 30 minutes. The supernatant were saved as total cytosolic protein extract.
Preparation Of Boiled Extract
Crude extract prepared as was incubated at 950C water bath for 7-10 minutes with constant stirring and cooled on ice. The precipitated protein was discarded following centrifugation and the remaining supernatant was saved as heat stable fraction.
Deae-Cellulose Choromatograph
Sample of 3ml volume were applied separately on to a DEAE-Cellulose column {4cm ´2mm} equilibrated with buffer A (20mM Tris acetate pH=7.6, 20mM NaCl, 0.1mMEDTA). After washing the column with buffer A until the absorbance of the eluate decreased to less than 0.025 at 280nm. Mixture of proteins bound as a yellow zone at top of column was eluted with a linear 20mM-500mM NaCl gradient in buffer A at a flow rate of 25ml/hour. Gradient volume used was 5 times the bed volume of mini column [4ml]. 50 fractions of 0.5ml volume of the eluate collected.
Nuclear Protein Extraction
Nuclear protein extract were prepared according to method of Zhu et-al (2001). To the nuclear pellets, ice cold high salt buffer containing [20mM Hepes, 25% Glycerol, 0.42M NaCl 0.2mM EDTA, 1.5mM MgCl2, 0.5mM PMSF) was added and mixed nuclei were incubated for 15 minutes and centrifuged at maximum speed for 1 minute. Supernatant were saved as nuclear protein extract.
Protein estimation
Protein concentrations at each step of the purification was determined by bradford method using bovine serum albumin as standard.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
SDS/page was carried out in 12% separating gel with a 5% stacking gel according to lammilli. The proteins were visualized by staining with 0.1% coomasie brilliant blue R250.
Western Blotting
Different protein samples were separated on 10% polyacrylamide gels and then transferred to a PVDF membrane (Himedia) for an hour at 75V in a transfer buffer containing (24mM Tris base, 0.2M Glycine 0.1% SDS, and 20% Methanol) . Non specific binding sites on the membrane was blocked using 3% bovine serum albumin in tris buffered saline pH-7.4 for overnight at 37C. Membrane was incubated with primary antibody (1m g/ml). After washing, antibody binding was visualized by alkaline phosphatase conjugated secondary antibody (1mg of ALP) with 5-Bromo-4chloro-3 itroblue tetrazolium as substrate. Color development was stopped by 20mM EDTA.
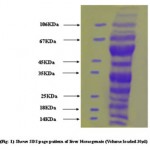 |
Figure 1
|
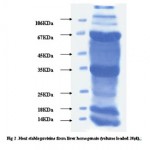 |
Figure 2
|
Fig-3 70KDa protein eluted at fractions 15-22 when 1ml of sample volume was applied to minicolumn of 4ml bed length anion exchange chromatography. Protein was fractionated using a linear gradient of 20-500mM NaCl and the protein peak of70KDa monomer eluted at 80mM.
 |
Figure 3
|
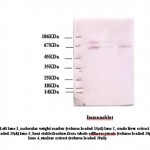 |
Figure 4
|
Western analysis of 70KDa protein
Equal amounts of protein (20μg/lane) from cell lysate prepared after indicated treatments were saperated by SDSpage and western blotted using anti Hsp70 polyclonal antibody (1μg/ml). A very prominent band was seen at the expected size of 70KDa nearly in all three fractions. However, light bands were found in heat stable fraction due to lower concentration of protein present.
References
- Chirico, W.J., Waters, M.G., and Blobel, G. 70KDa heat shock related proteins stimulate protein translocation into microsomes. Nature (1998) 332, 805-810.
- Deshaies, R., Koch, B., Werner- Wash burne, M., Craig, E.A., and Scheckman, R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptide. Nature (1988) 332, 800-805.
- Chiang, H.L., Terlecky, S. R., Plant, C.P., and Dice, J.F. A role for a 70-KDa heat shock protein in lysosomal degradation of intracellular proteins. Science (1989) 246, 382 – 385.
- Beckmann, R. P., Lovett, M., and Welch, W.J. Examining the function and regulation of Hsp70 in cells subjected to metabolic stress. J. Cell Biol.( 1992) 117, 1137 – 1150.

This work is licensed under a Creative Commons Attribution 4.0 International License.





