How to Cite | Publication History | PlumX Article Matrix
Aara Rifat1, S. A. Hajam1 and K. I. Andrabi1
1Department of Biochemistry, University of Kashmir, Kashmir - 190 006 India.
ABSTRACT: A heat stable protein (hsp) has been purified to homogeneity from sheep liver extract by heating to 95c followed by anion-exchange chromatography on DEAE-cellulose (DE-52).. The molecular weight of purified protein was found to be 35KDa
KEYWORDS: Hsp; DEAE; SDS-page; sheep liver
Download this article as:| Copy the following to cite this article: Rifat A, Hajam S.A ,Andrabi K.I. Purification of 35KDa heat stable protein (hsp) from sheep liver tissue by anion exchange chromatography. Biosci Biotechnol Res Asia 2008;5(2). |
Introduction
The enhanced synthesis of a few proteins immediately after subjecting cells to a stress such as heat shock was first reported for drosophila cells in 1974 (Tissieres 1974, Mitchell 1974, Tracy 1974)
Methodology
Preparation of Crude Liver Extract
Frozen sheep liver was cut into small pieces then homogenized in lysis buffer containing (10mM Tris acetate pH=7.5, 10mM NaCl, 1mMEDTA, 1mMPMSF) using a hand held homogenizer. The homogenate was centrifuged at 700g for 30 minutes Pellet was discarded and the supernatant recentrifuged at 700g for 30 minutes. The supernatant were saved as total cytosolic protein extract.
Preparation of Boiled Extract
Crude extract prepared as was incubated at 950C water bath for 7-10 minutes with constant stirring and cooled on ice. The precipitated protein was discarded following centrifugation and the remaining supernatant was saved as heat stable fraction.
Ion Exchange Chromatography
Anion-exchange chromatography was performed for further purification of proteins obtained at different saturations of ammonium sulfate.
Deae-Cellulose Choromatography
Dialyzed ammonium sulfate fractions were applied separately on to a DEAE-Cellulose column {4cm ´2mm} equilibrated with buffer A (20mM Tris acetate pH=7.6, 20mM NaCl, 0.1mMEDTA). After washing the column with buffer A until the absorbance of the eluate decreased to less than 0.025 at 280nm. Mixture of proteins bound as a yellow zone at top of column was eluted with a linear 20mM-500mM NaCl gradient in buffer A at a flow rate of 25ml/hour. Gradient volume used was 5 times the bed volume of mini column [4ml]. Fractions of 0.5ml of the eluate collected.
Protein estimation
Protein concentrations at each step of the purification was determined by bradford method using bovine serum albumin as standard.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
SDSpage was carried out in 12% separating gel with a 5% stacking gel according to lammilli. The proteins were visualized by staining with 0.1% coomasie brilliant blue R250.
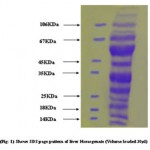 |
Figure 1
|
 |
Figure 2
|
Result
The boiled extract was adjusted to 15% w/v, with respect to acid, by addition of a cold solution of 100%w/v,trichloroacetic acid.After 30 minutes the precipitate was collected by centrifugation at 20,000g for 30 minutes. The precipitate was dissolved in minimal volume of 20mM Tris(pH 7.5) containing 2mM EDTA (Buffer B) by keeping constant pH 7.5 with the addition of 1N NaOH.This step was primarily used for concentrating the large volume of boiled extract and resulted in neither decrease in total protein .The suspension was applied to a column of DEAE-Cellulose equilibrated with Buffer.The haet stable protein HSP35 was first recovered in the 90-120mM NaCl eluate from DEAE Cellulose(DE-52column.The fractions containing protein eluted as a broad peak were pooled and easily identified by SDS-PAGE.
References
- Tissieres A, Mitchell,H.K and Tracy(1974) J.Mol. Biol.84,389.
- Ellins R.J(1990), Semin, Cell Biol,1, 1-72.

This work is licensed under a Creative Commons Attribution 4.0 International License.





