How to Cite | Publication History | PlumX Article Matrix
Theoretical Studies of The Contraction and Extension Effect in The B-Ring of TIBO Derivatives
Y. Belmiloud1,2, A. Kadari2, M. Brahimi2, D. Cherquaoui3, D. Villemin4 and A. Schmitzer5
1C. R. A. P. C., B. P. 248- Alger - 160 04 Alg?rie.
2L. P. C. T. C. I. Facult? de Chimie, U. S. T. H. B., BP 32 Al-alia ; Bab-Ezouar – Alger Algerie.
3Facult? des Sciences Semlalia, B. P. 2390,- Universit? Cadi Ayyad, Marrakech Maroc.
4ENSICAEN. LCMT, UMR CNRS, 6507, boulevard Mar?chal Juin, 14050 Caen France.
52900 Edouard Mont petit CP 6128 Succursale. Universit? Montr?al, Qu?bec Canada.
ABSTRACT: Some new non-nucleoside reverse transcriptase inhibitors are designed by contraction and extension of the seven-membered ring of TIBO to six- and eight-membered rings respectively using PM3, HF and DFT methods. Independently of calculations level, contracted seven-membered ring in C15 position, showed great similarity between the 3D geometry and the molecular properties of the TIBO and 6MRC15. Otherwise, the same ring when extend between N4 and C15 atoms showed also a great similarity between TIBO and 8MR (N4-C15). The latter molecule can enter easily in the allosteric cavity of NNRTIs. These two TIBO derivating compounds conserve the butterfly-like conformation can constitute a new class of anti-HIV.
KEYWORDS: HIV; Reverse Transcriptase; 7 ring TIBO; contraction and extension ofTIBO, PM3; HF; DFT
Download this article as:| Copy the following to cite this article: Belmiloud Y, Kadari A, Brahimi M, Cherquaoui D, Villemin D, Schmitzer A. Theoretical Studies of The Contraction and Extension Effect in The B-Ring of TIBO Derivatives. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Belmiloud Y, Kadari A, Brahimi M, Cherquaoui D, Villemin D, Schmitzer A. Theoretical Studies of The Contraction and Extension Effect in The B-Ring of TIBO Derivatives. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7113 |
Introduction
The treatment in HIV infection domain considers Non-Nucleoside Reverse Transcriptase (NNRTIs) as more significant Inhibitors. These inhibitors include a very large diversity of chemical compounds, as TIBO derivatives which are the main aim of our work. Although many physicochemical properties of NNRTIs can differ, many theoretical studies seem to establish that these inhibitors possess a common three-dimensional feature which has a rigid butterfly-like configuration.
In the literature, some new TIBO-like derivatives have been designed by contraction and extension of the b-ring of TIBO derivatives from seven to six and eight-membered ring, respectively. In this work, we will examine the effect of modified structures on butterfly-like configuration, geometrical, electronic and physicochemical properties by using various quantum mechanics methods. Computational calculations shown in this work are in agreement with literature. Therefore, the resulting molecules can be considered as new candidates for synthesis of anti-HIV inhibitors.
Human Immunodeficiency virus (HIV) is a retrovirus which can be replicated in a human host cell. It appears to preferentially attack helper T cells. In fact, the viral particle becomes active only when it is in contact with a cell [1]. The treatment of the acquired immunodeficiency syndrome (AIDS) caused by HIV is one of the most challenging medical problem. This major issue is mainly due to the fact that the polymerase of virus HIV makes hundred more errors than a polymerase of a bacterium. So, a vaccine or a drug which can eliminate or block the action of the virus is more difficult to be found [2].
To fight against this virus, many researches were directed towards the chemotherapy and the design of drugs decimating the HIV or blocking its replication. HIV Reverse Transcriptase enzyme (RT) appeared to be a key target of anti-HIV therapy, because it catalyzes an essential step in virus replication. This enzyme is the responsible in the conversion of viral genomic RNA into proviral DNA which later integrates the host genome [2,3]. There are two types of RT inhibitors: the Nucleoside (NRTIs) and the Non-Nucleoside (NNRTIs). NRTIs act on the catalytic site of the reverse transcriptase enzyme, preventing DNA synthesis, whereas NNRTIs bind non-competitively to a hydrophobic site close to the catalytic site, forcing the enzyme to adopt an inactive conformation [4, 5]. The NNRTIs are much less toxic than the NRTIs [6, 7].
Generally, the NNRTI compounds contain a carbonyl or thiocarbonyl group, an extra lipophilic site and a methyl group [8]. Though, many physicochemical properties of NNRTIs inhibitors can differ, many theoretical studies have shown clearly that these inhibitors possess a common three-dimensional feature which has a rigid butterfly-like configuration in the internal cavity of the allosteric area of the enzyme [9-13]. The degree of butterfly-like configuration depends on the overall shape parameters, the polarizability and the lipophilicity of the molecule [13]. According to its structural diversity, NNRTIs interact with different amino acid residues of the allosteric pocket. However the number of amino acid residues interacting with these inhibitors has an important relation with the angle formed by the two wings of the inhibitor butterfly-like configuration. Moreover, this angle can be closely correlated with the drug affinity for the enzyme and the probability of drug-resistance development [9-13]. Therefore, a great importance have to be given to this configuration in the conception of new inhibitors to HIV reverse transcriptase.
TIBO compounds and its derivatives that belong to NNRTIs, have been efficient to inhibit RT (Figure 1). Similarely to all the other NNRTIs structures, the TIBO inhibitors can be located in the Binding Pocket of RT and adopt a butterfly-like conformation [14].
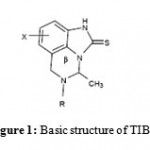 |
Figure 1: Basic structure of TIBO.
|
Some calculations [15-16] was carried out AM1 study of some new compounds resulting from the contraction and extension of the β- diazepine ring of TIBO derivatives, from seven- to six- and eight-membered rings, respectively (Figure 2 and 3). This work revealed that some six- and eight-membered rings derivatives bond to the receptor better than the seven-membered ring derivatives.
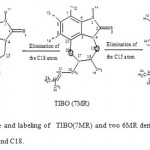 |
Figure 2: Structure and labeling of TIBO(7MR) and two 6MR derivatives obtaind by contaction in C15 and C18.
|
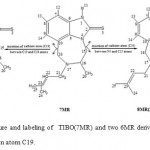 |
Figure 3: Structure and labeling of TIBO(7MR) and two 6MR derivatives obtaind by insertion of carbon atom C19.
|
Because this work presents several important results based only on semi-emperical approach, we aim to confront them to more precise technique calculations. Therefore new structures presenting butterfly-like configuration has been studied.. Geometrical, electronic and some pertinent physicochemical properties were calculated using various theoretical approaches and codes.
Computational methods
The calculations discussed in this work have been done at the Austin Model one (AM1), Hartree-Fock (HF), and DFT levels using a standard Gaussian 98 program package [17] with 6-31G* basis sets for the ab initio and DFT levels. The computations carried at DFT level [18-22] using the hybrid method B3LYP. A vibrational analysis has been performed on the HF and DFT/B3LYP and indicates that all optimized structures correpond to minima on the potential energy surface confiremed by the absence of any imaginary frequency.
In addition, physicochemical properties such as molar refractivity, ovality, volume and LogP were calculated using Chem-3D program. However, other properties as chemical hardness (h) [23], chemical potential (m) [23] and global electophilicity (w) [24] were obtained using DFT results.
Results and discussion
The background molecular structure of TIBO derivatives is formed from a seven-membered diazepine ring (b-ring) fused to a bicycle-aromatic moiety (figure 1). In order to propose new inhibitors, two modifications were made on the seven-membered ring, by eliminating or adding a carbon atom [15, 16]. The contraction of b-ring [15], produced by eliminating of a carbon atom in position 15 or 18, was lead to two new compounds containing a six-membered ring (Figure 2). We will adopt the following notation: 7MR designs a seven-membered ring compound., 6MRC15 and 6MRC18 design six-membered ring compounds produced by eliminating of C15 and C18, respectively.
However, the extension of the seven-membered ring to eight-membered rings[16], produced by insertion of a carbon atom (C19) between N4 and C15, or C10 and C18 atoms, was lead to two other compounds 8MR(N4-C15) and 8MR(C10-C18), respectively (Figure 3).
Note that the insertion of C19 between C15 and C16 atoms or C18 and N17 atoms gives the same compounds.
Contraction effect in b-ring
In the binding pocket of reverse transcriptase, TIBO structures, as all NNRTI inhibitors, adopt a butterfly–like conformation (figure 4), in which dimethylallyl group (Wing I) interacts with Pro95, Tyr181, Tyr188, Gly190 and Trp229 amino acid residues of P66. However, the benzodiazepinone group (Wing II) interacts with Lys101, Lys103, Val106, Phe227, His235, Pro236, and Tyr318 of P66. Residues Leu100 and Leu234 of P66 interact with both Wings I and II from the top, Val179 from the front, and Tyr188 from the back of the butterfly [14].
![Figure 4 : Representation of NNRTI’s butterfly-like configuration in interaction with aminoacid residues of P66 [2].](https://www.biotech-asia.org/wp-content/uploads/2016/03/Vol_5-no2_theo_vill_fig4-150x150.jpg) |
Figure 4 : Representation of NNRTI’s butterfly-like configuration in interaction with aminoacid residues of P66 [2].
|
All calculations have shown that TIBO molecular structure yields as a butterfly-like conformation which is in agreement with literature [15, 25-28]. The seven-membered ring contraction in position C15 leads to a butterfly-like geometry, but it is comparable to that calculated in NNRTIs like the nevirapine which shown an angle of 108 to 115° [10].
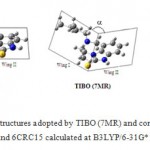 |
Figure 5: Butterfly-like structures adopted by TIBO (7MR) and contracted homologues MRC18 and 6CRC15 calculated at B3LYP/6-31G* level.
|
Otherwise, the ring contraction in position C18 was lead to a slight planarity of the two wings (Figure 5). Then, the butterfly-like geometry hasn’t been kept for this structure. Indeed, the geometrical optimization of this compound shows planar torsion angles N4C5C10N17 and C20N17C10C5. For this structure, the nitrogen N17 and carbon C20 (dimethylallyl group) are in the same plane carrying the fragment C10C5N4C15. In contrary, the same nitrogen N17 in the TIBO and 6MRC15 is outside of plane. We can conclude that the position of the nitrogen N17 in 6MRC18 structure differs from those of TIBO and 6MRC15. Consequently, the position of the C20 atom linked to N17, will be different. These results are confirmed by the values of the α angle (figure 5), formed by the two wings in the TIBO derivatives. These calculated values of α, at different levels of theories, are summarized in the following table.
Table 1: PM3, HF and DFT Calculated angle limited between wings I and II of TIBO, 6MRC15 and 6MRC18 butterfly-like conformations.
|
Method |
a (°) | ||
| C15 atom | TIBO | C18 atom | |
| PM3
HF/6-31G* B3LYP/6-31G* |
113.3
114.5 113.0 |
111.43 110.22 110.45 | 160.8
169.22 157.04 |
For these reasons, 6MRC18 cannot have a butterfly-like geometry and no have anti-HIV activity.
The interaction of NNRTIs with the allosteric site of the RT adopts key-lock mode. The molecules are selected according to their shape to get into the receptor site cavity [29]; in this case, the calculation of molecular volume (V) has shown that 6MRC15 owns an average volume when compared to TIBO and 6MRC18:
V (TIBO) > V (6MRC15) > V (6MRC18).
The different results show that 6MRC15 possesses a more oval structure than TIBO and 6MRC18. We notice that contraction effect in position C15 leads to a decrease of 1.67% in volume and more over the 6MRC15 structure becomes more oval. It is known that the compounds which have weak sizes and which can set the best interactions have chances to be more active than the big sized compounds [29]. Indeed, the big molecules can have access a difficult to the receptor site, unlike the small molecules which enter more easily in the allosteric site cavity of the NNRTIs which has a W form [30].
The table 2 summarizes all calculated electronic and physicochemical properties for various studied structures.
Table 2: Clculated of selected electronic and physicochemical properties of TIBO, 6MRC15 and 6MRC18 using 6-31G* on DFT method.
| DFT/6-31G* | X=H | ||
| C15 atom | TIBO | C18 atom | |
| E(HOMO)
E(LUMO) E(HOMO-LUMO) Molar Refractivity Ovality V LogP h m w.104 |
-0.0919
0.0797 -0.1716 86.69 1.491 588.67 3.62 0.0858 0.0061 2.168 |
-0.093
0.079 -0.172 92.28 1.465 598.92 3.53 0.086 0.007 2.841 |
-0.082
0.093 -0.175 88.16 1.471 552.10 4.00 0.087 0.005 1.728 |
A different computational level shows a great similarity between 3D geometrical and molecular properties of TIBO and 6MRC15 which confirms those found by ref. [15].
Extension effect in b-ring
The extension of the seven-membered ring between the N4 and C15 atoms leads to a butterfly-like conformation. The calculated angle, separating the two wings shows 108.55 degrees at DFT/B3LYP/6-31G* method (Figure 6). This is close to 110.45 degrees, angle obtained for TIBO structure at the same level. Otherwise, this angle remains comparable to that found in NNRTIs, such as the nevirapine (108 to 115 degrees) [10].
On the other hand, the extension of b-ring between C10 and C18 atoms doe not lead to a butterfly–like geometry. In fact, we notice that this compound is composed of three planes having a chair form, as shown in Figure 6.
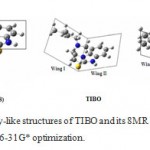 |
Figure 6: butterfly-like structures of TIBO and its 8MR derivatives through B3LYP/6-31G* optimization.
|
In this issue, the volume V of the two eight-membered ring structures was calculated and compared to that of TIBO as follows:
V8MR (N4-C15) > V8MR (C10-C18) > VTIBO
This shows that 8MR (N4-C15) compound has a similar structure to TIBO, but exhibits a bigger volume with a less oval structure. We should remember that the rayon a0 is equal to 5.49 Å that remains inferior to the one obtained in the TIBO when we substitute the hydrogen (H14) carried by the C9 carbon, by the group (-OC2H5). This latter has a ray of a0=5.60 Å and its experimental anti-HIV activity is 7.02 [8, 31-33]. We conclude that 8MR (N4-C15) can enter in the allosteric cavity of NNRTIs.
In addition to the volume and Ovality other electronic and physicochemical properties are presented in the following table.
Table 3: Selected calculated electronic and physicochemical properties of TIBO and 8MR compounds.
| B3LYP/6-31G* | 8MR(C10-C18) | TIBO | 8MR(N4-C15) |
| E(HOMO)
E(LUMO) E(HOMO-LUMO) Molar Refractivity Ovality V LogP h m w.102 |
-0.193
-0.022 -0.171 97.04 1.411 692 3.95 0.085 0.107 6.75 |
-0.093
0.079 -0.172 92.28 1.465 598.92 3.53 0.086 0.007 2.841.10-2 |
-0.189
-0.021 -0.168 97.15 1.425 711 3.77 0.084 0.105 6.56 |
We can conclude that the 8MR (N4-C15) has a great similarity of 3D geometry than TIBO. Thus, the molecules resulting from the extension of the 7-membered ring between N4 and C15 atoms can constitute a new class of anti-HIV compounds.
Conclusion
Our calculation focus on some new non-nucleoside reverse transcriptase inhibitors are designed by contraction and extension of the seven-membered ring of TIBO to six- and eight-membered rings respectively using PM3, HF and DFT methods. When we contract the seven-membered ring in C15 position, these results found at all level of calculations, have shown that there is a great similarity between the 3D geometry and the molecular properties of the TIBO and 6MRC15. Otherwise, when we extend the same ring between N4 and C15 atoms, results have shown that there is, also, a great similarity between TIBO and 8MR (N4-C15); this molecule can enter easily in the allosteric cavity of NNRTIs. So, our results confirm those found in the literature. These two resulting compounds which conserve the butterfly-like conformation can constitute a new class of anti-HIV compounds.
References
- S.P. Goff, J. Acq. Immun. Def. Synd. 3 (1990) 817.
- De Clercq, E. J. Med. Chem. 38 (1995) 2491.
- A. A. Toropov, A. P. Toropova, I. V. Nesterov, O. M. Nabiev. Theochem 640 (2003) 175–181.
- Renato F. Freitas, Sérgio E. Galembeck. Chemical Physics Letters. 423 (2006)131-137.
- De Clercq, E. J. Med. Chem. 48 (2005)1297.
- De Clercq. Il Farmaco. 54 (1999) 54, 26.
- Kalyan Das, Jianping Ding, Yu Hsiou. J. Mol. Biol. 264 (1996) 1085-1100.
- Rajni Garg, Satya P. Gupta, Hua Gao, Mekapati Suresh Babu, Asim Kumar Debnath, and Corwin Hansch. Chem. Rev. 99 (1999) 3525-3601
- Mui, P. W.; Jacober, S. P.; Hargrave, K. D.; Adams, J. J. Med. Chem. 1992, 35, 201.
- Schafer, W.; Friebe, W.-G.; Leinert, H.; Mertens, A.; Poll, T.; von der Saal, W.; Zilch, H.; Nuber, B.; Zeigler, M. L. J. Med. Chem. 1993, 36, 726.
- Ding, J.; Das, K.; Moereels, H.; Koymans, L.; Andries, K.; Janssen, P. A. J.; Hughes, S. H.; Arnold, E. Nature Struct. Biol.1995, 2, 407.
- Mager, P. P.; De Clercq, E.; Takashima, H.; Ubasawa, M.; Sekiya, K.; Baba, M. Walther, Eur. J. Med. Chem. 1996, 31, 701.
- Mager, P. P. Drug Des. Discovery 1996, 14, 241.
- Ding, J., Das, K., Moereels, H., Koymans. L.,Andries, K., Janssen,P. A. J., Hughes,S. H., Arnold, E., Nature Struct. Biol. 1995, 2, 407-415.
- B. Hemmateenejad, S. Mohammad Hossein Tabaei and Fatemeh Namvaran. J. of Molecular Structure. 732 (2005) 39-45.
- B. Hemmateenejad, S. M. H. Tabaei, F. Namvaran. Journal of the Iranian Chemical Society(2007),4(4),481-489.
- Gaussian 98, Revision A.9, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E.
- Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C.
- Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, Cioslowski, Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W.Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head- Gordon, E. S. Replogle, and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1998.
- A. D. Beke, Phys. Rev. A38:3098 (1988)
- C. Lee, W. Yang, R. G. Parr, Phys. Rev. B37:785(1988).
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, J. Phys. Chem. 98:11623(1994).
- H. B. Schlegel, J. Comp. Chem. 3:214(1982).
- H. B. Schlegel, Theor. Chim. Acta. 66:33(1984).
- W. J. Hehre, R. F. Stewart and J. A. Pople, J. Chem. Phys. 51 (1969) 2657.
- Parr, R. G. and Yang, W. J. Am. Chem. Soc., 106 (1984) 4049-4050.
- J. B. Collins, P. v. R. Schleyer, J. S. Binkley and J. A. Pople. J. Chem. Phys. 64(1976) 5142.
- R. G. Parr . J. Chem Phys 68 (1978) 3801.
- M. B. K. Smith, M. L. Lamb, J. Tirado-Rives, W. Jorgensen, C. J. Michejda, S. K. Ruby, Smith Jr., Protein Eng. 13 (2000) 413.
- M. LL. Barreca, A. Carotti, A. Carrieri, A. Chimirri, A. M. Monforte, M. P. Calace, A. Rao, Bioorg. Med. Chem. 7 (1999) 2283.
- M. A. L. Ericsson, J. Pitera, P. A. Kollman, J. Med. Chem. 42 (1999)868.
- R.H. Smith Jr., W. Jorgensen, J. Tirado-Rives, M. L. Lamb, P. A. J. Janssen, C. J. Michejda. M. B. K. Smith, J. Med. Chem. 41 (1998) 5272.
- Latifa Douali, Didier Villemin and Driss Cherqaoui. Int. J. Mol. Sci. 5(2004) 548-55.
- Mager, P. P. Drug Des. Discovery. 14 (1996) 225-239.
- Kukla, M. J., Breslin, H. J., Diamond, C. J., Grous, P. P., Ho, C. Y., Miranda, M., Rodgers, J. D., Sherril, R. G., De Clercq, E., Pauwels, R., Andries, K., Moens, L. J., Janssen, M. A. C., Janssen, P. A. J. J. Med. Chem. (1991), 34, 3187.
- Breslin, H. J., Kukla, M. J., Ludovici, D. W., Mohrbacher, R., Ho, W., Miranda, M., Rodgers, J. D., Hitchens, T. K., Leo, G., Gauthier, D. A., Ho, C. Y., Scott, M. K., De Clercq, E., Pauwels, R., Andries, K., Janssen, M. A. C., Janssen, P. A. J. J. Med. Chem. (1995), 38, 771.
- Ho, W., Kukla, M. J., Breslin, H. J., Ludovici, D. W., Grous, P. P., Diamond, C. J., Miranda, M., Rodgers, J. D., Ho, C. Y., De Clercq, E., Pauwels, R., Andries, K., Janssen, A. C., Janssen, P. A. J. J. Med. Chem. (1995), 38, 794. (b) Cramer, R. D., III, Bunce, D., Patterson, D. E., Frank, I. E. Quant. Struct.-Act. Relat. (1988), 7, 18.
- Chromatographic chiral separations. Edited by Morris Zief and Laura J. Crane. V(40).
- C. Peng, P. Y. Ayala, H. B. Schlegel, J. Comput. Chem. 17 (1996) 49.
- A. Peeters, C. Van Alsenoy, J. Dillen and H. J. Geise. J. Mo. Struct. 333 (1995) 99-110.
- I. K. Boessenkool and J. C. A. Boeyens, J. Cryst. Mol. Struct. 10 (1980) 11.
- Y. C. Liaw, Y. G. Gao, H. Robinson and A. H. J. Wang. J. Am. Chem. Soc. 113 (1991) 1857.
- Handbook of chemistry and physics. David R. Lide. Editor in Chief 73RD Edition 92-93.

This work is licensed under a Creative Commons Attribution 4.0 International License.





