How to Cite | Publication History | PlumX Article Matrix
Total antioxidant power and free radical scavenging capacity of some medicinal plants
P. K. Rao1, V. Bobbarala2, D. Bhaskar Rao1, Ch. Ravi Kiran1, K. V. Raghava Rao1 and T. Raghava Rao1
1Department of Biochemistry, College of Science & Technology, Andhra University Visakhapatnam - 03 India.
2For U Biosciences, A/4A, Park lane Residency, East point colony, Visakhapatnam, A. P - 530 017 India.
Corresponding Author E-mail: koti_au@yahoo.co.in
ABSTRACT: Current research is now directed towards finding naturally occurring antioxidants of plant origin. The present study was aimed to assess the In vitro antioxidant activity of methanolic leaf extracts of some medicinal plants by carrying the determination of Total Antioxidant power (FRAP Assay) and Free radical scavenging capacity (DPPH Assay) and results were compared with well established antioxidants like Ascorbic Acid and Butylated Hydroxy Toluene(BHT). Our results clearly showed that all the plants of present study showed potent Total Antioxidant power and Free radical scavenging capacity. Of all the plants tested, the highest total antioxidant power and free radical scavenging capacity was shown by X. mekongensis, B. cylindrica and E. agallocha with nearly equal to antioxidant capacity to stable antioxidants like BHT and Ascorbic acid. This data will be useful in estimating the total pharmacological and phytochemical properties of these plants.
KEYWORDS: Reactive oxygen species; DPPH Radical; Ascorbic acid Equivalents; Free radical scavengers
Download this article as:| Copy the following to cite this article: Rao P. K , Bobbarala V, Rao D. B, Kiran C. R, Rao K. V. R , Rao T. R. Total antioxidant power and free radical scavenging capacity of some medicinal plants. Biosci Biotechnol Res Asia 2008;5(2). |
Introduction
Reactive oxygen in particular, free radicals are considered to induce oxidative damage in biomolecules and to play an important role in aging, cardiovascular diseases, cancer, and inflammatory diseases (1-3). In addition, they are also well known to be major causers of material degradation and food deterioration (4). Consequently, antioxidants are now known to be prospective protective or therapeutic agents. In the past few years, addition of synthetic antioxidants has begun to be restricted because of their health risks and toxicity (5). The importance of exploiting natural antioxidants from various sources and replacing synthetic antioxidants with natural ingredients has attracted increasing attention. Several natural compounds from plants exhibit antioxidant / radical scavenger properties are preferred because they are safe and environmental friendly. It has become clear that the direct free radical scavenging effect and membrane protection play an important role in the action mechanism of several old established drugs. Several herbs and spices have been reported to exhibit antioxidant activity, including rosemary, sage, thyme, nutmeg, turmeric, white pepper, chili pepper, ginger, and several Chinese medicinal plants extracts (6-9). The aim of the present work was to prove the phytotherapeutical significance of some popular medicinal plants on the basis of their antioxidant activity due to their influence on pathological free radical reactions. The medicinal plants of present study includes Borreria hispida (BH) belongs Rubiaceae, Bruguiera cylindrica (BC) and Ceriops decandra (CD) belongs to Rhizophoraceae, Eugenia bracteata (EB) belongs to Myrtaceae, Excoecaria agallocha (EA) belongs to Euphorbiaceae, Glycyrrhiza glabra (GG) belongs to Fabaceae, Picrorhiza kurrow (PK) belongs to Scrophulariaceae, Trianthima decandra (TD) belongs to Aizoaceae, Xylocarpus mekongensis (XM) belongs to Meliaceae. The reason for selection is that, these plants have a wide range of applications in traditional medicines and are involved in the treatment of various skin diseases, liver disorders, cancer, HIV, cough, asthma, diarrohea, dysentery, ulcers and controlling of blood pressure etc,. In addition, some of these plants were also reported to be the potent antioxidants. Hence, in the present study, we made an attempt to study the total antioxidant power and radical scavenging capacity of methanolic extracts of these plants. This data will be used to estimate the phytochemical and antioxidant intake of the local population and to understand the therapeutic uses of these plants.
Materials and Methods
Diphenyl picryl hydrazyl (DPPH) was obtained from Himedia laboratories Pvt. Ltd, Mumbai, India. Ferric Chloride, 2,4, 6-tri pyridyl-s-triazine, Butylated hydroxy toluene (BHT) were obtained from Sisco research laboratories Pvt. Ltd., Mumbai, India. All other chemicals used were of analytical grade obtained from commercial sources.
Plant collection and preparation of the extraction
The plants are collected from the near by hilly region, Paderu and coastal region of Visakhapatnam, Andhra Pradesh, India and authenticated by Dr. M. Venkayya, Associate professor, Dept.of Botany, Andhra University,Visakhapatnam, Andhrapradesh. Plant leaves were cleaned with deionized water and dried at 50ºC for 24 hours. The dried plants were ground and then sieved 80mesh. The dried leaves were ground to a powder using a milling machine. The dried powder was then weighed and extracted with methanol, with reflux on a water bath at 40ºC for three consecutive days. The extracts were filtered and evaporated under vacuum to dryness with a rotary evaporator and then placed in an oven at 60ºC until constant weight was obtained and the solutions were prepared with a concentration 1mg/ml using methanol.
Total Antioxidant power
Ferric reducing ability of Plasma (FRAP) Assay
The total antioxidant potential of a sample was determined using the ferric reducing ability of plasma i.e., FRAP assay as a measure of antioxidant power by Benzie and Strain (10). The assay was based on the reducing power of a compound (antioxidant). A potential antioxidant will reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+); the latter forms a blue complex (Fe2+/TPTZ), which increases the absorption at 593 nm. Briefly, the FRAP reagent was prepared by mixing acetate buffer (300µM, pH 3.6), a solution of 10 µM TPTZ in 40 µM HCl, and 20µM FeCl3 at 10:1:1 (v/v/v). The reagent (300 µM) and sample solutions (10 µL) were added to each well and mixed thoroughly. The absorbance was taken at 593 nm after 10 min. Standard curve was prepared using different concentrations of FeCl3. All solutions were used on the day of preparation. The results were corrected for dilution (e.g.1000ml) and expressed as ascorbic acid equivalents (µmoles/ml) or FRAP units. All determinations were performed in triplicates.
Free radical scavenging capacity
Di Phenyl Picryl Hydrazyl radical scavenging assay (DPPH Assay)
The DPPH assay was carried out as described by Cuendet et al., (11). 5.0 ml of DPPH solution (0.004%) in methanol was added to 50 µl of plant extract. After 30 min of incubation at 370C, the absorbance was read against control at 517 nm. Ascorbic acid and BHT were used as positive controls. Percentage of inhibition = (absorbance of control –absorbance of test / absorbance of control) x100. All determinations were performed in triplicate.
Results and Discussion
Recently much attention has been focused on reactive oxygen species and free radicals, which play an important role in the genesis of various diseases such as inflammation, cataract, liver cirrhosis and ischemia/reperfusion injury (12). Herbal drugs containing radical scavengers are gaining importance in the prevention and treatment of such diseases (13). Hence the present study is focused on the determination of Total antioxidant power by FRAP Assay and DPPH Assay.
Total Antioxidant power
Ferric reducing ability of Plasma (FRAP) Assay
The FRAP assay measures the reduction of Fe3+ (ferric iron) to Fe2+ (ferrous iron) in the presence of antioxidants. Because the ferric-to-ferrous ion reduction occurs rapidly with all reductants with half reaction reduction potentials above that of Fe3+/Fe2+, the values in the FRAP assay will express the corresponding concentration of electron-donating antioxidants (10). We elected to use the FRAP analysis for several reasons. The FRAP assay is the only assay that directly measures antioxidants or reductants in a sample. The other assays are indirect because they measure the inhibition of reactive species (free radicals) generated in the reaction mixture, and these results also depend strongly on the type of reactive species used. The FRAP assay, in contrast, uses antioxidants as reductants in a redox-linked colorimetric reaction. Furthermore, the other assays, but not the FRAP assay, use a lag phase type of measurement. This has been difficult to standardize in previous experiments and has generated varying results among different laboratories. In the FRAP assay, pretreatment is not required, stoichiometric factors are constant and linearity is maintained over a wide range. The results also indicated that the total antioxidant power of the plant extracts was determined by the FRAP method was observed to be highest in X. mexoeugensis with 1601 FRAP units followed by B. cylindrica(1471 FRAP Units), E. agallocha (1237 FRAP Units), E. bracteata (1140 FRAP Units), G. glabra (862 FRAP Units), T.decandra (720 FRAP Units), P. kurrow (645 FRAP Units), B. hispida (600 FRAP Units) and C. decandra (360 FRAP Units) These results were presented graphically in Figure.1.
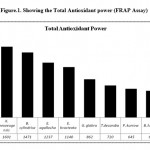 |
Figure 1: Showing the Total Antioxidant power (FRAP Assay).
|
 |
Figure 2: Showing the Free radical scavenging capacity (DPPH Assay).
|
Free radical scavenging capacity
Di Phenyl Picryl Hydrazyl radical scavenging assay (DPPH Assay)
The free radical scavenging capacity of methanolic leaf extracts of the plants of present study were tested by its ability to bleach the stable DPPH radical. Antioxidants react with DPPH, which is a stable free radical, and convert it to 1,1-diphenyl-2- (2,4,6- trinitrophenyl) hydrazine(14). The degree of decolourization indicates the scavenging potentials of the antioxidant compounds. This assay provided information on the reactivity of the test compound with a stable free radical, since its odd electron with DPPH gives strong absorption band at 517 nm in visible spectroscopy (deep violet colour). As this electron becomes paired off in the presence of a free radical scavenger, the absorption vanishes and the resulting decolourization is stoichiometric with respect to the number of electrons taken up. All the plant extracts showed significant free radical scavenging activity by inhibiting DPPH radical with the percentage of inhibition was observed to be maximum in stable and well established antioxidant BHT (76.2%) Ascorbic acid (71.2 %) followed by X. mexoeugensis with 70.3% B. cylindrica (62%), E. agallocha (51.4%), E. bracteata (51%), G. glabra (49 %), P. kurrow (46.1%), T.decandra (33.7%), B. hispida (28.4%) and least is observed in C. decandra (24.5 %). These results were presented graphically in Figure.2. We therefore suggest that these plant extracts may act as free radicals scavengers and may react with free radicals to convert them to more stable products and terminate radical chain reaction (15). Our results clearly shown that plants with high Total Antioxidant power showed maximum Free radical scavenging capacity which was almost equivalent to well established antioxidants like Ascorbic acid and Butylated Hydroxy Toluene (BHT).
References
- T. Finkel, N. J. Holbrook., Nature., 408, 239-47 (2000) .
- Bauerova, A. Bezek., Gen. Physiol. Biophys.,18, 15 (1999).
- Halliwell., Lancet 322., 721, (1994).
- D. Kathie., Eur. J. Clin. Nutr., 45, 759 (1993).
- Buxiang, M. Fukuhara., Toxicology., 122, 61,(1997),.
- Kikuzaki H, Usuguchi J, Nakatani N., Chem Phar Bull ., 39: 120 (1991).
- Jitoe A, Masuda T, Tengah IGP., J. Agr Food Chem., 8: 1337(1992).
- Kikuzaki H, Nakatani N., J Food Sci., 58: 1407-1410 (1993).
- Lee SE, Hwang HJ, Ha JS. Life Sci 73: 167-179 (2003).
- Benzie I.F.F and Strain J.J., Anal Biochem., 239: p70-76 (1996)
- Cuendet M, Hostettmann K, Potterat O., Helv. Chim. Acta., 80: p1144 -1152(1997).
- Halliwell B., Nutr. Reviews., 55: p522-544(1994).
- Prajakta V Desai, Raju R Wadeka, Girish H Kedar and Kalpana S Patil., Int. J. of Green Pharmacy., 2(1):p31-33(2008).
- Ozgen, M., Reese, R. N., Tulio Jr., A. Z., Scheerens, J. C., & Miller, R., J. Agr Food Chem., 54, 1151-1157. (2006).
- Duh PD, Yen GC., Food Chemistry., 60: 639–645 (1997).

This work is licensed under a Creative Commons Attribution 4.0 International License.





