How to Cite | Publication History | PlumX Article Matrix
Osama A. H. Abu-Zinadah1 and Suzan B. S. Abdu*2
1Biological Sciences Department King Abdulaziz University, Jeddah Saudi Arabia *
2Department of Zoology, Girls collage, Jeddah Saudi Arabia.
Corresponding Author E-mail: oaboznada@kau.edu.sa
ABSTRACT: The anthracycline antibiotic Doxorubicin is one of the most effective antitumour agents used to treat human malignancies. Long term treatment with DOX is limited by its hepatotoxicity. The purpose of the present investigation was to evaluate the hepatotoxic effect of the drug when used alone or combined with the iron-chelating agent Dexrazoxane (DXZ) as protective effect. Twenty four adult male rats were divided into 3 groups: Control group, group2A, Doxorubicin 1 week (DOX 1wk), group2B, Doxorubicin 2 weeks (DOX 2wk) and group3, Doxorubicin +Dexrazoxane (DOX+DXZ 1 wk. and 2 wk.). Ultrastructural damage showed hepatocytes injury after 1week and more severed after 2 wk as Hepatocyte cell appears with vacuolated with damage mitochondria structures and vacuolated in nucleus. Liver cell disappear some cytoplasm organelles such as rough endoplasmic reticulum , smooth endoplasmic reticulum and lysosomes.
KEYWORDS: Ultrastructre; TEM; toxic effect; protective effect; hepatocyte; doxorubicin; dexrazoxane
Download this article as:| Copy the following to cite this article: Abu-Zinadah O. A. H, Abdu S. B. S. Ultrastructural Studies on the Changes Induced by Toxic Effect of Doxorubicin on Rat Hepatocyte and Protective Role of Dexrazoxane. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Abu-Zinadah O. A. H, Abdu S. B. S. Ultrastructural Studies on the Changes Induced by Toxic Effect of Doxorubicin on Rat Hepatocyte and Protective Role of Dexrazoxane. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7144 |
Introduction
Doxorubicin, a quinone-containing anthracycline antibiotic, is an important agent against a wide spectrum of human neoplasms. However, its toxicity limits usage in cancer chemotherapy (Singal, et al., 1987; Fadillioglu, et al., 2003). It has been shown that free radicals are involved in doxorubicin-induced cytotoxicities (Quiles, et al., 2002;Yagmurca, et al., 2004; Gajewski et al., 2007). The chemical structure of doxorubicin causes the generation of free radicals and the induction of oxidative stress that correlates with cellular injury (Saad, 2001). Doxorubicin causes an imbalance between free oxygen radicals (ROS) and antioxidants. The disturbance in oxidant antioxidant systems results in tissue injury that is demonstrated with lipid peroxidation and protein oxidation in tissue (Karaman, 2006). Endogenous antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase enzyme in liver tissue can limit the effects of ROS but quickly become overwhelmed by large quantities of ROS (Bagchi et al., 1995 and Durk et al., 1998). Several studies have demonstrated that together with inflammatory processes, free radicals, oxidative stress and lipid peroxidation are frequently associated with liver damage induced by toxic agents such as doxorubicin. Regulation of these mediators has been considered a therapeutic necessity to prevent doxorubicin induced toxicities in various organs (Yagmurca, et al., 2007; Abd El-Aziz, et al., 2001; Fadillioglu, et al., 2004).
Doxorubicin increases the urinary excretion of a number of aldehydes (acetaldehyde, formaldehyde and malondialdehyde) with concomitant increase in hepatic lipid peroxidation (Bagchi et al., 1995 and Pierscinski et al., 1994). With respect to the liver, other effects include doxorubicin induced hepatic mRNA induction (Fardel, et al., 1997), nuclei changes (Merski et al., 1976 ), reduction in glutathione (Rapozzi et al., 1999), increases in microsomal UDP-glucuronosyltransferase activity (Lear et al, 1992), inhibition of oxidative phosphorylation (Shinozawa et al, 1991) and inhibition of NADPH cytochrome c. reductase activities (Mungikar and Gothoskar, 1985). The involvement of metal ions in doxorubicin toxicity has led to the development of various cytoprotective agents such as the bisdioxopiperazine compound dexrazoxane (ICRF-187) (Hasinoff et al., 1991; Kvetina et al., 1997 and Zima et al. 1998).
Liver cancer, in particular, hepatocellular carcinoma (HCC), is one of the major cancer killers (Liovet, 2005). There is not first line option for patients with advanced HCC. Systemic doxorubicin provides partial responses, but no clear survival advantages, and well-known treatment-related complications (Liovet, 2005).
The indications of oxidative injury of mitochondria include membrane lipid peroxidation, inhibition of respiration and oxidative phosphorylation, decrease mitochondria ATPase activity, breaking of mitochondrial DNA helix and slow down mitochondrial DNA synthesis (Ji and Mitchell, 1994). Thus, protective strategies to prevent cytotoxicity without reducing the antitumour effect of anthracyclines including : augmentation of endogenous antioxidant defences (De Atley et al.,1999) alpha- tocopherol (Geetha, 1993) and iron chelating agents as Dexrazoxane ( ICRF-187) .
Initial studies on experimental animals (Torre et al., 1999) and human showed that Dexrazoxane (ICRF-187) is a promising agent to decrease doxorubicin cytotoxicity because it neither interferes with anthracycline distribution, metabolism or excretion nor it reduces the antitumour potency of anthracyclines. In addition, it imposes limited damage on mitotically active tissues such as bone marrow, liver, testes and G.I.T.epithelia.
The aim of the present study was evaluate ultrastructurally changes in the liver of the normal rats and rats treated with Doxorubicin as toxic effect and Dexrazoxane as protective effect.
Materials and Methods
Animal
The present study was carried out on 24 adult male albino rats weighing 150-250 g each and aged 2 months. They were kept in clean ventilated cages and fed on a commercial laboratory diet.
Drugs and treatment schedules
The animals were divided in 3 groups: Control group (8 rats), Doxorubicin group (DOX group) 8 rats subdivided into 2 subgroups (A,B) each 4 rats according to the duration after DOX injection. DOX: 1 week (group 2A), DOX: 2 weeks (group2B) and group receiving DOX + DXZ for the same duration 1week and 2 weeks. DOX was administrated as a single an ip dose injection 1.5ml/150gm and Dexrazoxane (DXZ) was administrated 30 minutes prior to injection of DOX and the dose administrated was 3.3ml/150gm.as a single an ip dose. These doses of the used drugs are more or less equivalent to the human doses used in the management of acute leukaemia and malignant lymphomas.
Electron microscopy
The liver tissues were first washed in deionized water to separate blood then cut liver tissues into small cubes of 1-2mm³ and immersed in perfusion fixative for two hours. Fixation was continued in 2.5% glutaraldehyde in 0.1 M Sodium cacodylate buffer pH 7.4. After rinsing in cacodylate buffer, specimens were postfixed in aqueous 1% OsO4 for two hours at 4Cº. Washing in the same buffer about three times , 5min each. Specimens were dehydrated in a graded series of 30 to 100% ethanol-100% propylene oxide and then infiltrated in a 1:1 mixture of propylene oxide- epoxy resin for one hour, then embedded in pure epoxy resin, followed by hardening at 60°C for two days. Ultrathin (60-70nm) sections were collected on 200-mesh copper grids and stained with 0.5% aqueous uranyl acetate for one hour , followed by 0.5 % lead citrate for 20 min. by Leica autostainer. Sections were photographed using a JEOL JEM 1011 transmission electron microscope at 80 kV with Gatan CCD Camera.
Results
General examination
The animals received DOX demonstrated general weakness, loss of appetite and weight, diarrhea, redness around nose and mouth, yellowish colouration and loss of hairs in certain areas of the body.
Electron microscopic Examinations
Control group
Electron micrographic examination of the control rat liver sections showed the normal ultrastructure. The hepatocytes contained rounded nucleus and surrounded by the ground cytoplasm which containing different cell organelles including, mitochondria which are round and oval outlines with their mitochondrial ridges and rough and smooth endoplasmic reticulum , Golgi-apparatus were present near the nucleus. Glycogen particles could be also detected in the cytoplasm of the hepatocytes. (Fig.1).
Group 2
 |
Figure 1
|
Electron Microscopy ultrastreuctural of hepatocyte the subgroup A (DOX 1wk) shows the nucleus had changed in shape, the outer and inner membranes fused together and shows oval in shape. The chromatin material was condensed into small deeply staining. Hepatocyte cell appears with vacuolated in cytoplasm and Rough endoplasmic reticulum (RER) adhering and rounded with nucleus. liver cell appears with dense rounded and oval mitochondria. Lysosomes like small rounded dense stained granules were observed in the cytoplasm masses. (Fig. 2)
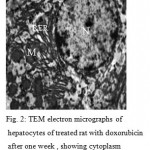 |
Figure 2
|
Electron Microscopic examinations of subgroup 2B (DOX 2 wk) observes the nucleus had changed in shape, the outer and inner membranes fused together and shows oval in shape, with small condensed patches of chromatin and disappear nuclei. Hepatocyte cell appears with vacuolated with damage mitochondria structures. Liver cell disappear some cytoplasm organelles such as rough endoplasmic reticulum , smooth endoplasmic reticulum, Golgi apparatus. Lysosomes like small rounded dense stained granules were observed in the cytoplasm masses. (Fig. 3 ).
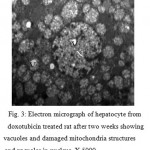 |
Figure 3
|
Group 3
Ultrastrurally examination the liver cell , subgroup 3A (DOX +DXZ 1wk)) showing slightly changes in creastia of mitochondria and appear different cytoplasm organelles like normal cell (Fig. 4).
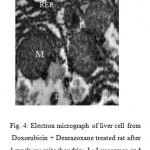 |
Figure 4
|
The electron microscopic examination of the liver of rat which treated with doxorubicin and dexrazoxane after two weeks showing normal structure of hepatocytes included round nucleus ; round and oval mitochondria; rough and smooth endoplasmic reticulum; lysosomes and glycogen. (Fig. 5).
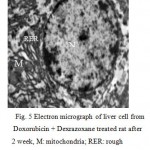 |
Figure 5
|
Discussion
Doxorubicin showed cardiotoxic and hepatotoxic effects in animals. It is known that antibiotics such as DOX cause weight loss (Durak et al., 1998; Kalender et al., 2002). For this reason, their prolonged use and over dosage cause death. These drugs cause disruption in basal metabolism by showing toxic effect especially in liver and hearth tissues. Our electron microscopic findings showed that DOX caused hepatotoxic effect.
Doxorubicin (DOX) is a widely used chemotherapeutic drug for human hepatocellular carcinoma. A major limitation to its effectiveness is the development of multidrug resistance of cancer cells (Irena Manov et al. 2007). Doxorubicin also accumulates in the liver, which might damage hepatocytes. doxorubicin impairs liver function in rats with liver fibrosis and cirrhosis.
Murt, et al., 2007 found that, The tissue of the doxorubicin group showed some histopathological changes such as necrosis, hepatocyte degeneration, sinusoidal dilatation, hemorrhage and vascular congestion and dilatation. In the doxorubicin plus erdosteine group, histopathological evidence of hepatic damage was markedly reduced. Two different ways of free radical formation by DOX have been described. The first way implicates the formation of a semiquinone free radical by the action of several NADPH-dependent reductases that produce a one-electron reduction of the DOX to the corresponding DOX semiquinone. In the presence of oxygen, redox cycling of DOX-derived quinone–semiquinone yields superoxide radicals. In the second way, DOX free radicals come from a non enzymatic mechanism that involves reactions with iron (Kalender et al., 2005 and Yagmurca et al., 2007). For example, Fe3+ reacts with DOX in a redox reaction after which the iron atom accepts an electron and a Fe2+ DOX free radical complex is produced. This iron–DOX complex can reduce oxygen to hydrogen peroxide and other active oxygen species (Quiles et al., 2002). Damage at the cell level by oxidants is attenuated by antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase.
Doxorubicin has several acute and chronic side effects, particularly a dose-depended myocardial injury, which can lead to a potentially congestive heart failure (Singal et al., 1998). Other tissues like the kidneys, brain, liver and the skeletal muscles, are also affected by Doxorubicin (Kalender et al., 2005). Adriamycin, anticancer drug, forms a complex with DNA, inhibiting the synthesis of both DNA and RNA. Its metabolism also results in ROS formation (Halliwell and Gutteridge, 1989 and Trush and kensler, 1991).
Electron microscopy studies showed that DOX cause pathological changes in hepatocytes. This effect was seen in mitochondria, nucleus and endoplasmic reticulum of liver cells. However, DOX + DXZ treated group showed no pathological changes in liver cells. As a result, in our study by electron microscopy ultrastructure indicated that Doxorubicin caused hepatotoxic effect was prevented by Dexrazoxane.
Acknowledments
The author’s are grateful to Dr. Nabeel Zahed and Dr. Salim El- Hamidy for their help with Transmission Electron Microscope at the Electron Microscopy Unit, Biological Sciences Dept., Faculty of Science , King Abdulaziz University.
References
- Singal PK, Deally CM, Weinberg LE., Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol., 99, 817-828 (1987).
- Fadillioglu E, Erdogan H, Sogut S, Kuku I., Protective effects of erdosteine againsedoxorubicin-induced cardiomyopathy in rats. J. Appl Toxicol., 23: 71-74 ( 2003).
- Quiles, J.L., Huertas, J.R., Battino, M.,Mataix, J., Ramirez-Tortosa, M.C., Antioxidant nutrients and adriamycin toxicity. Toxicology. 180, 79–95 (2002).
- Yagmurca M, Erdogan H, Iraz M, Songur A, Ucar M, Fadillioglu E., Caffeic acid phenethyl ester as a protective agent against doxorubicin nephrotoxicity in rats. Clin Chim Acta, 348, 27- 34 (2004).
- Gajewski E, Gaur S, Akman SA, Matsumoto L, van Balgooy JN, Doroshow JH. Oxidative DNA base damage in MCF-10A breast epithelial cells at clinically achievable concentrations of doxorubicin. Biochem Pharmacol. 73, 1947–56 (2007).
- Saad SY, Najjar TA, Al-Rikabi AC., The preventive role of deferoxamine against acute doxorubicin induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 43, 211-218 (2001).
- Karaman A, Fadillioglu E, Turkmen E, Tas E, Yilmaz Z., Protective effects of leflunomide against ischemia reperfusion injury of the rat liver. Pediatr Surg Int., 22: 428-434 (2006).
- Bagchi, D., Bagchi, M., Hassoun, E.A., Kelly, J., Stohs, S.J., Adriamycin-induced hepatic and myocardial lipid-peroxidation and DNA-damage, and enhanced excretion of urinary lipid metabolites in rats. Toxicology., 95: 1–9 (1995).
- Durak, , Ozturk, H.S., Kavutcu, M., Birey, M., Yel, M., Guven, T., Olcay, E., Kacmaz, M., Canbolat, O., Protective role of antioxidant vitamins on adriamycin-induced free radical production and cardiotoxicity in guinea pigs. Cancer Res. Ther. Cont., 5:133–141 (1998).
- Yagmurca M, Bas O, Mollaoglu H, Sahin O, Nacar A, Karaman O, Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch Med Res., 38: 380–385 (2007).
- Abd El-Aziz MA, Othman AI, Amer M, El-Missiry MA., Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J. Appl. Toxicol., 21: 469-473 (2001).
- Fadillioglu E, Oztas E, Erdogan H, Yagmurca M, Sogut S, Ucar M., Protective effects of caffeic acid phenethyl ester on doxorubicininduced cardiotoxicity in rats. J Appl., Toxicol., 24: 47-52 (2004).
- Pierscinski G, Drzewoski J, Nowak D., The in uence of doxorubicin and 4 -epi- doxorubicin on lipid peroxidation inmouse heart, lungs and liver. Part II. Pol J Pharmacol., 46: 55–59 (1994).
- Fardel O, Lecureur V, Daval S, Corlu A, Guillouzo A Up-regulation of P-glycoprotein expression in rat liver cells by acute doxorubicin treatment. Eur J Biochem., 246:186–192 (1997).
- Merski JA, Daskal I,BuschH., Effects of adriamycin on ultrastructure of nucleoli in the heart and liver cells of the rat. Cancer Res. 36, 1580–1584 (1976).
- Rapozzi V, ComelliM, Mavelli I, SentjurcM, Schara M, Perissin L, Giraldi T Melatonin and oxidative damage in mice liver induced by the prooxidant antitumor drug, adriamycin. In Vivo, 13: 45–50 (1999).
- Lear L, Nation RL, Stupans I, Effects of cyclophosphamid e and adriamycin on rat hepatic microsomal glucuronidation and lipid peroxidation. Biochem Pharmacol,. 44: 747–753 (1992).
- Shinozawa S, Gomita Y, Araki Y, Effect of aclarubicin and doxorubicin on rat liver mitochondrial oxidative phosphorylation . Physiol Chem Phys Med NMR., 23: 101–106 (1991).
- Mungikar AM, Gothoskar BP, Depression of mouse liver microsomal mixed function oxidase enzymes by adriamycin. Toxicol Lett., 29:17–23 (1985).
- Hasinoff BB, Reinders FX, Clark V., The enzymatic hydrolysisactivation of the adriamycin cardioprotective agent -1,2-bis(3,5- dioxopiperazinyl-1-yl)propane. Drug Metab Dispos., 19: 74–80 (1991).
- Kvetina J, Grossmann V, Svoboda Z, Safarova M., Preclinical comparison of bis- diketopiperazine-propan e (dexrazoxane) and bisdiketopiperazine- ethane (antimet) on the adriamycin-cardiotoxi c effect. Neoplasma., 44: 97–99 (1997).
- Zima T, Tesar V, Crkovska J, Stejskalova A, Platenik J, Teminova J, Nemecek K, Janebova M, Stipek S, ICRF-187 (dexrazoxan ) protects fromadriamycin-induced nephrotic syndrome in rats. Nephrol Dial Transplant. 13: 1975–1979 (1998).
- Liovet JM., Updated treatment approach to hepatocellular carcinoma. J Gastroenterol., 40: 225–35 ( 2005).
- Ji, L. L., and Mitchell, E. W., Effects of Adriamycin on heart mitochondrial function in rested and exercised rats. Biochem. Pharmacol., 47: 877-885 (1994).
- De Atley, S.M., Aksenov, M. Y., Aksenov, M. V., Harris, A. B., Hanley, R., Cole Harper, P., Carney, J, M., Butterfield, D. A., Antioxidants protect against reactive oxygen species associated with adraimycin- treated cardiac myocytes. Cancer lett., 136: 41-46 (1999).
- Geetha, A., Influence of α-tocopherol on doxorubicin-induced lipid peroxidation, swelling and thiol depletion in rat heart mitochondria. Ind. J Exp. Biolol., 31: 297- 298 (1993).
- Torre, P. D, Anthony R. I., Claudio B. A.P., Donatella M., M. R.and Guy M., Cardioprotection by dexrazoxane in rats treated with doxorubicin and paclitaxel. Biomedical and life Science., 44, 2: 138-142 (1999).
- Kalender, S., Kalender, Y., Ates, A., Yel, M., Olcay, E., Candan, S., Protective role of antioxidant Vitamin E and catechin on idarubicin-induced cardiotoxicity in rats. Braz. J. Med. Biol. Res., 35: 1379–1387 (2002).
- Irena M., Yulia B., Anat E., Meital M., Oded L., and Theodore C., High-Dose Acetaminophen Inhibits the Lethal Effect of Doxorubicin in HepG2 Cells: The Role of P-glycoprotein and Mitogen-Activated Protein Kinase p 44/42 Pathway. Journal of Pharmacology And Experimental Therapeutics, DOI:10.1124/jpet.107.121772 (2007)
- Murat Y, Orhan B, Hakan M, Onder S, Ahmet N, Ozcan K and Ahmet S, Protective effects of Erdosteine on Doxorubicin- induced Hepatotoxicity in Rats. Medical research, 38: 380 – 385 (2007).
- Kalender Y, Yel M, Kalender S., Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology., 209: 39–45 (2005).
- Singal PK, Iliskovic N., Doxorubicin induced cardiomyopathy. N Engl J Med; 339: 900 – 905 (1998).
- Halliwell B and Gutteridge JMC., free radicals in biology and medicine. Oxford University Press, New York, 543 (1989).
- Trush MA and Kensler TW, Role of free radicals in carcinogen activation. In: Sies H, ed. Oxidative stress: Oxidants and Antioxidants. Acadimaic Press, London. 277- 318 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





