How to Cite | Publication History | PlumX Article Matrix
Biochemical and Antimicrobial Studies of the Genus Jatropha Excisa
D. S. V. G. K. Kaladhar1*, K. V. V. V. Satyanarayana2 and I. Bhaskar Reddy3
1Department of Biochemistry and Bioinformatics, GITAM Institute of Science Gitam University India.
2Department of Chemistry, Gitam Institute of Science Gitam University India.
3Department of Biochemistry and Bioinformatics, GITAM Institute of Science, GITAM University India.
Corresponding Author E-mail: dkaladhar@gmail.com
ABSTRACT: Jatropha excisa is an ornamental plant, which is employed as a source of medicine with different antimicrobial activities and differentiation in organization of metabolites. Metabolite analysis of this plant was analyzed phytochemically and screened against different microorganisms responsible for various infections. Qualitative analysis of various primary and secondary metabolites was analyzed by Thin Layer Chromatography. Present report has shown more number of amino acids in wet leaves, carbohydrates in seeds and dry leaves, lipids in seeds and dry leaves, and secondary metabolites in flowers. The antimicrobial activity of the fresh methanolic leaf extract was tested against standard strains of bacteria and fungi using agar well diffusion method. Plant fresh methanolic leaf extract of 0.1mg/ml concentration produced different zone formation on Microorganisms. The zone formation was high in gram-negative bacteria such as Escherichia coli (16 mm), Micrococcus leuteus (15 mm) and Proteus mirabilis (14 mm) compared to the gram-positive bacteria such as Streptococcus faecalis (12 mm), Klebsiella pneumoniae (11 mm) and Bacillus subtilis (12 mm). The extracts were found to be effective against bacteria (11-16 mm) and yeast (15 mm) than multicellular, filamentous fungi such as Aspergillus niger and Penicillium chrysogenum (9-11mm).
KEYWORDS: Jatropha excisa; TLC; Metabolites Antimicrobial activity
Download this article as:| Copy the following to cite this article: Kaladhar D. S. V. G. K, Satyanarayana K. V. V. V, Reddy I. B. Biochemical and Antimicrobial Studies of the Genus Jatropha Excisa. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Kaladhar D. S. V. G. K, Satyanarayana K. V. V. V, Reddy I. B. Biochemical and Antimicrobial Studies of the Genus Jatropha Excisa. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=8343 |
Introduction
Due to rapid studies in biotechnology and synthetic chemistry, the debate on the use of plants possessing several medicinal properties could be assessed due to their therapeutic value1. Medicinal substances found in plants are the products of natural metabolic processes. Each species has its own genetic structure that governs the presence of chemical components or bioactive molecules2. Medicinal herbs are moving from fringe to mainstream use; as a greater number of people endeavor to opt for herbal formulations over the allopathic compounds, since these are devoid of side effects and cost effective3.
Plants from the genus Jatropha, natively occur in India, Africa, North America, and the Caribbean. Jatropha is a morphologically diverse genus having 160-175 species of trees, shrubs, rhizomatous subshrubs, and geophytes, distributed primarily in seasonally dry tropics. Related species not withstanding to Geographical changes have high gene exchange within wide limits under artificial conditions; phylogenetic relationships may be inferred by cross ability of the taxa4, 5. Jatropha excisa Griseb. which has been reported in 1874, is a shrub6 characterized by the frequent occurrence of milky sap.
Considerable attention has been focused on identifying naturally occurring chemo preventive substances7. Since ancient times, plants have been an exemplary source of medicine. Ayurveda and other Indian literature mention the use of plants in treatment of various human ailments. India has about 45,000 plant species and among them, several thousands have been claimed to possess medicinal properties8.
Medicinal plants are the most important sources of life saving drugs for a majority of the world’s population. Biotechnological tools are important to select, multiply and to conserve the critical genotypes of medicinal plants9. Due to large demand of phytomedicines, plant tissue culture techniques are now being used to obtain large number of diverse medicinal plants, monitoring their respective secondary metabolites10, 11.
Materials and Methods
Collection of plant materials
The plant material is largely found in Gajuwaka region of Visakhapatnam District. Whole plant was collected and the experiments were conducted during April – June 2008 (summer season).
Preparation of plant extracts
Freshly collected samples were thoroughly washed with distilled water separately. 100 g of each plant materials (seeds, fresh flowers, fresh roots, fresh and dried leaves) were separately homogenized with 100 ml of Methanol and squeezed through three layers of cheesecloth to remove larger particles. The collected homogenate is used as sample for metabolite analysis.
Metabolite Analysis
Reagents for amino acids
Amino acids standards: 20 amino acids (0.25 % in (HCl 0.5 M in ethanol)).
Silica gel in TLC was of “Lichrosolv” grade from Merck, India.
Solvent mixture: (Butanol: Acetic acid: Water = 4:1:1)
Spraying reagent: 0.3% Ninhydrin in Butanol containing 3 ml of Acetic acid.
Reagents for carbohydrates
Carbohydrate standards: sucrose, starch, Galactose, lactose and xylose (1 % standard in 10% Isopropinol).
Silica gel in TLC was of “Lichrosolv” grade from Merck, India.
Solvent mixture: (Ethyl acetate: Isopropyl alcohol: water = 130:57:2.3)
Spraying reagent: Aniline diphenyl Amine reagent: Mix 5 volumes of 1% Aniline in Acetone and 5 volumes of 1% Diphenyl amine (DPA) in Acetone with 1 volume of 85% hydrogen phosphate.
Reagents for lipids
Silica gel in TLC was of “Lichrosolv” grade from Merck, India.
Solvent mixture: Chloroform: methanol: Water = 65:25:4
Spraying reagent: 30% concentric sulphuric acid
Reagents for secondary metabolite analysis
Silica gel in TLC was of “Lichrosolv” grade from Merck, India.
Solvent mixture: Chloroform: Acetone: Ammonium hydroxide = 30:70:2
Spraying reagent: 5% concentrated H2SO4 (in ethanol)
Procedure
Plates were made with silica gel by mixing about 30 gm of Silica gel (G60) with 60ml of double distilled water and the contents are vigorously mixed until the gel is uniformly dispersed. This procedure should be completed with in 4 minutes after addition of water. Then the slurry is poured into Stahl’s mechanical spreader adjusted to 0.2 mm thickness. Using spreader the gel is layered on the glass plate and dried at room temperature for few minutes. Subsequently the plates are placed in an oven, kept at 100°C for 30 minutes and allowed to cool. (Stahl 1969)
Into respective developing chambers, solvent mixture was poured to a depth of about 10 mm. Cover the internal walls of the chambers with filter paper and wet it with the solvent system (or solvent mixture) used in that chamber. Close the lid and the tank is left for 10-15 minutes for saturation with solvent vapours. Softly, mark every TLC plate with a pencil line about 1.5 cm from the bottom. Subsequently make a mark every 1.5 cm from left to right. These marks will be used to apply for standards and samples. Hold the micropipette vertically and briefly touch the filled end to the pencil mark line. The samples prepared from field grown leaves (methanoic extract) were applied on the TLC plate in 10µl quantities. Hold the micropipette vertically and briefly touch the filled end to the pencil mark. Liquid should flow from the micropipette on to the plate to form a spot. Similarly standards are also applied on TLC plate in 10µl quantities. After loading the samples and standards, the plates are allowed to air dry. Once the spots have been dried, lower the plate carefully into the chamber. Close the lid and monitor the movement of solvent up the plate. When the solvent has advanced at least 15 cm, remove the plate from the chamber, mark the solvent front with a pencil, and allow all solvents to evaporate. Spray the plate with spraying reagent, let it dry, and heat it inside the oven at 110 °C for 15 minutes. The plates are removed and calculate the Rf values.

Antimicrobial Activity
Preparation of plant extracts
Freshly collected leaves were thoroughly washed with distilled water separately. 100 g of plant material was homogenized with 100 ml of Methanol. The homogenate were thoroughly squeezed through three layers of cheesecloth to remove larger particles and then centrifuged at 10,000 xg at 4°C for 20 min. The supernatant was collected and the centrifugation process was repeated for 3 times at 10,000 xg at 4°C for 20 min. The final extract was filtered through whattman’s filter paper no.1. The methanol present in the methanolic extract was evaporated under reduced pressure (Buchi vaporator) to yield the residue. The residue thus obtained was suspended in Dimethylsulfoxide (DMSO) to concentration of crude extract of 0.1mg/ml. The extracts were kept at -20°C before use for maximum 24 hours.
Microorganisms
Six Bacterial and three fungal cultures were obtained from Microbial Type Culture Collection (MTCC), Chandigarh, India and preserved in deep freezer at Dept. of Microbiology, GITAM University, Visakhapatnam, India. The cultures employed for experimentation are Escherichia coli (MTCC No.118), Micrococcus leuteus (MTCC No 106) and Proteus mirabilis (MTCC NO.425) of gram-negative bacteria, Streptococcus faecalis (MTCC No.439), Bacillus subtilis (MTCC NO.121) and Klebsiella pneumoniae (MTCC NO.109) of gram-positive bacteria and Saccharomyces cerevisiae (MTCC No. 463), Aspergillus niger (MTCC No.281) and Penicillium chrysogenum (MTCC NO. 5108) of fungi.
Maintenance of Microorganisms
The above bacterial cultures were maintained on Mueller–Hinton Agar (MHA) and fungal cultures were maintained on Sabouraud Dextrose Agar (SDA) at 4°C temperature until used for the study. Before use, the Bacterial and Fungal cultures were revived in Mueller Hinton Broth (MHB) for Bacteria and Sabouraud Dextrose Broth (SDB) for Fungi.
Antimicrobial assay
Zone Method is carried out for antimicrobial assay. MHA for bacterial growth and SDA for fungal growth was weighed and mixed in distilled water based on the composition. The media was autoclaved for 20 min. at 121°C (15 lbs pressure) and cooled to 45°C. The bacterial and fungal cultures with optical density of 0.6 were taken and 50 ml of inoculum was added per 500ml of MHA for bacteria and yeast, SDA for fungi. 20 ml of the media was poured in each plate and was kept for solidification. By using gel puncture, 8 mm diameter wells had been made in the plate for the addition of plant extract at 0.1mg/ml concentration. Fresh leaf extract was added into the well and incubated for 24-48 hours for bacteria and yeast and 2 days for fungi. The zone of inhibition can be calculated based on the results obtained by scale reading. With the scale the zone formed on the plate was calculated and was tabulated.
Results
The qualitative analysis of various primary and secondary metabolites was analyzed by TLC results. The Results of TLC were tabulated and listed in Tables 1 and 2.
Table 1: Rf values: Metabolite analysis of methanolic extracts of J.excisa.
| Seed | Flower | Root | Fresh Leaf | Dry leaf | |
| Amino acids | 0.739 | 0.739 | 0.626 | 0.479 | 0.577 |
| 0.967 | 0.934 | 0.577 | 0.723 | ||
| 0.756 | 0.861 | ||||
| 0.894 | |||||
| Carbohydrates | 0.370 | 0.481 | 0.518 | 0.370 | 0.333 |
| 0.688 | 0.703 | 0.444 | 0.644 | ||
| 0.925 | 0.948 | 0.925 | 0.792 | ||
| 0.962 | 0.866 | ||||
| 0.925 | |||||
| Lipids | 0.764 | 0.785 | 0.75 | 0.714 | 0.65 |
| 0.857 | 0.928 | 0.785 | 0.785 | 0.764 | |
| 0.942 | 0.964 | 0.964 | 0.964 | 0.907 | |
| 0.942 | 0.964 | ||||
| Secondary metabolities | 0.862 | 0.689 | 0.896 | 0.931 | Nil |
| 0.931 |
Table 2: TLC analysis with Standard Rf values.
| Amino acids | Carbohydrates | ||
| Standard | Rf | Standard | Rf |
| A ALA | 0.46 | A-Sucrose | 0.42 |
| C CYS | 0.51 | B-Starch | 0.05 |
| D ASP | 0.68 | C-Galactose | 0.54 |
| E GLU | 0.60 | D-lactose | 0.10 |
| F PHE | 0.87 | E-xylose | 0.70 |
| G GLY | 0.35 | ||
| H HIS | 0.28 | ||
| I ILE | 0.95 | ||
| K LYS | 0.17 | ||
| L LEU | 0.65 | ||
| M MET | 0.89 | ||
| N ASN | 0.15 | ||
| P PRO | 0.53 | ||
| Q GLN | 0.50 | ||
| R ARG | 0.37 | ||
| S SER | 0.47 | ||
| T THR | 0.57 | ||
| V VAL | 0.55 | ||
| W TRP | 0.92 | ||
| Y TYR | 0.85 | ||
The methanolic extract of samples and standards had shown various spots on silica gel of TLC plates, and Rf values had been reported. The seeds, flowers, roots, wet leaves and dry leaf extracts had shown 2 (Rf values 0.739 and 0.967), 2 (Rf values 0.739 and 0.934), 1 (Rf value 0.626), 4 (Rf values 0.479, 0.577, 0.756 and 0.894), and 3 (Rf values 0.577, 0.723 and 0.861) amino acids respectively. 4 (Rf values 0.370, 0.688, 0.925 and 0.962), 1(Rf value 0.481), 3(Rf values 0.518, 0.703 and 0.948), 3(Rf values 0.370, 0.444 and 0.925) and 5(Rf values 0.333, 0.644, 0.792, 0.866 and 0.925) carbohydrates had been shown by seeds, flowers, roots, wet leaves and dry leaf extracts respectively.Analysis on seeds, flowers, roots, wet leaves and dry leaf extracts to lipids had shown 3(Rf values as 0.764, 0.957, 0.942), 3 (Rf values 0.785, 0.928 and 0.964), 3 (Rf values of 0.75, 0.785 and 0.964), 3(Rf values 0.714, 0.785 and 0.964), and 4 (Rf values 0.65,0.764,0.907 and 0.964) respectively. The seed, flower, root, and wet leaf extracts has shown 1 (Rf value 0.862), 2(Rf values 0.689 and 0.931), 1(Rf value 0.896), and 1(Rf value 0.931) secondary metabolite/s respectively.
Based on standards used, seeds contain isoleucine and xylose, flowers contain tryptophan, roots contains glutamic acid and xylose, fresh leaves contain serine, threonine, phenylalanine and sucrose, and dry leaves contains threonine and phenylalanin.
Figure 1 shows more number of amino acids in wet leaves, more number of carbohydrates in seeds and dry leaves, more number of lipids in seeds and dry leaves, and more number of secondary metabolites in flowers.
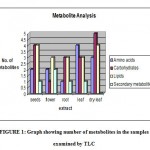 |
Graph 1: Graph showing number of metabolites in the samples examined by TLC.
|
The ethnobotanical screening test of plant leaf extracts of Jatropha excisa in methanol as solvent against both human and plant pathogenic bacteria and fungi using zone technique was depicted in Table 3 and Figure 2. Two antibiotics (penicillin and Streptomycin) were conducted as standards shown different zones of inhibition and was listed in Table 4
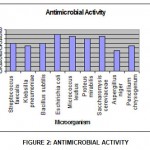 |
Graph 2: Antimicrobial Activity.
|
Table 3: Antimicrobial activity: Methanolic leaf extract of Jatropha excisa showing zone of inhibition on various microorganisms
| Microorganism
|
Zone of growth inhibition in mm
|
|
| Bacteria | ||
| Streptococcus faecalis | 12 | |
| Klebsiella pneumoniae | 11 | |
| Bacillus subtilis | 12 | |
| Escherichia coli | 16 | |
| Micrococcus leuteus | 15 | |
| Proteus mirabilis | 14 | |
| Fungi | ||
| Saccharomyces cerevisiae | 15 | |
| Aspergillus niger | 09 | |
| Penicillium chrysogenum | 11 | |
Table 4: Antimicrobial Activity Penicillin and Streptomycin as standards used as positive control.
|
Microorganism |
Zone of inhibition (in mm) | |
| Penicillin | Streptomycin | |
| Streptococcus faecalis | 12 | 26 |
| Klebsiella pneumoniae | 14 | 13 |
| Bacillus subtilis | 14 | 26 |
| Escherichia coli | 18 | 17 |
The methanolic leaf extracts of wild plants had exhibited antimicrobial activity and the Zone of inhibition was calculated based on the results obtained. The zone formation was high in Gram negative bacteria such as E.coli (16 mm), M.luteus (15 mm) and P.mirabilis (14 mm) compared to the gram-positive bacteria such as S.fecalis (12 mm), K.pneumonia (11 mm) and B.subtilis (12 mm). The extracts were found to be more effective against bacteria (11-16 mm zone formation) and yeast (15 mm zone formation) rather than multicellular, filamentous fungi (9-11mm zone formation).
Discussion
The quest for plants with medicinal properties continue to receive attention as scientists survey plants, particularly of ethnobotanical significance, for a complete range of biological activities, which range from antibiotic to antitumor. Thus far, plants have provided western medicine with an abundance of drugs and treatments for a variety of health problems12.
To define and describe the future tasks of phytomedicinal research in the new millennium, an analysis not only on the current state of development of phytomedicinal research but also on chemo synthetic pharmaceutical research was required. One advantage of phytotherapy is the availability of a wide group of medicinal drugs and preparation s that had been used over the centuries almost exclusively on the basis of empirical evidence. A reservoir of around 300,000 plant species exists, of which only about 30 percent had been investigated scientifically, inclusively the herbs and preparations of Chinese, Indian, South American and African traditional medicines13.
There are more number of amino acids in wet leaves, carbohydrates in seeds and dry leaves, lipids in seeds and dry leaves and secondary metabolites in flowers. Based on standards used, seeds contain isoleucine and xylose., flowers contain tryptophan., root contains glutamic acid and xylose., fresh leaves contain serine, threonine, phenylalanine and sucrose., dry leaves contain threonine and phenylalanin.
The methanolic leaf extracts of wild plants had exhibited antimicrobial activity and the Zone of inhibition was calculated based on the results obtained. The extracts were found to be more effective against bacteria (11-16 mm zone formation) and yeast (15 mm zone formation) rather than multicellular, filamentous fungi (9-11mm zone formation). The zone formation was high in gram-negative bacteria, such as E.coli (16 mm), M.luteus (15 mm) and P.mirabilis (14 mm) compared to the gram-positive bacteria S.fecalis (12 mm), K.pneumonia (11 mm) and B.subtilus (12 mm).
Acknowledgment
The authors acknowledge the support of the department of Biochemistry, GITAM University in providing necessary facilities in carrying out this work.
References
- Jitendra P. Srivastava, John Lambert and Noel Vietmeyer, Medicinal Plants: Rescuing a Global Heritage, RS 180 .C5 L174, 1 (1997).
- Thomas S. C. Li, Medicinal Plants: Culture, Utilization & Phytopharmacology, CRC Press, 1 (2002).
- Dubey N.K, Rajesh kumar and Pramila tripathi, Global promotion of herbal medicine: India’s opportunity, Current Science, 86(1), 37-41 (2004).
- Bijan Dehgan, Comparative Anatomy of the Petiole and Infrageneric Relationships in Jatropha (Euphorbiaceae), American Journal of Botany, 69(8), 1283-1295 (1982).
- Bijan Dehgan, Phylogenetic Significance of Interspecific Hybridization in Jatropha (Euphorbiaceae), Systematic Botany, 9(4), 467-478 (1984).
- Abh. Konigl, Jatropha excisa Griseb., Ges. Wiss. Gottingen., 19, 94 (1874).
- Surh Y, Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances, Mutat Res., 428(1-2), 305-327 (1999).
- Grover J.K, Yadav S. and Vats V, Medicinal plants of India with anti-diabetic potential, Journal of Ethnopharmacology, 81(1), 81-100 (2002).
- Debnath, Mousumi, Malik, C. P., and Bisen, P. S, Micropropagation: A Tool for the Production of High Quality Plant-based Medicines, Current Pharmaceutical Biotechnology, 7(1), 33-49 (2006).
- Merkle SA and Nairn CJ, Hardwood tree biotechnology. In Vitro Cellular and Developmental Biology – Plant, 41(5), 602–619 (2005).
- Rajasekharan PE, Ganeshan S, and Bhaskaran S, Conservation of Endangered Medicinal Plants; Challenges and Optiona, Indian J. Pl, Genet. Resour., 14, 296-297 (2001).
- Nishanta Rajakaruna, Cory S. Harris and G.H.N. Towers, Antimicrobial Activity of Plants Collected from Serpentine Outcrops in Sri Lanka, Pharmaceutical Biology, 40(3), 235–244 (2002).
- Zohara.Yaniv and Uriel Bachrach, Handbook of Medicinal Plants, Haworth Press, 3-4 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.





