How to Cite | Publication History | PlumX Article Matrix
Anjali Janbandhu and M. H. Fulekar*
Environmental Biotechnology Laboratory, Department of Life Sciences, University of Mumbai, Santacruz (E), Mumbai - 400 098 India.
ABSTRACT: The petrochemical industry waste disposal site located at Nagpur, Maharashtra has been studied for the characterization of waste and to identify the potential microorganism from the microbial consortium. In the present study physico-chemical as well as biological parameters including microbial consortium present in the contaminated soil were examined. In order to identify potential microorganism from the consortium, the organic compounds with special reference to naphthalene was exposed to microbial consortium at increasing concentration viz. 25, 50, 75, and 100 mg/l. The single colony of microorganism was found capable of degrading naphthalene up to 100 mg/l, when naphthalene was provided as a sole source of carbon. This bacterium was identified as “Achromobacter insolitus” using 16S rDNA sequencing and phylogenetic tree. This organism can be used for bioremediation of organic compounds such as naphthalene.
KEYWORDS: Bioremediation; naphthalene; Achromobacter insolitus
Download this article as:| Copy the following to cite this article: Janbandhu A, Fulekar M. H. Characterization of PAH Contaminated Soil for Isolation of Potential Microorganism Capable of Degrading Naphthalene. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Janbandhu A, Fulekar M. H. Characterization of PAH Contaminated Soil for Isolation of Potential Microorganism Capable of Degrading Naphthalene. Biosci Biotechnol Res Asia 2008;6(1). Available from: https://www.biotech-asia.org/?p=8088 |
Introduction
The petrochemical waste generated from refineries and petrochemical industries contains mixture of organic and inorganic compounds including complex Polycyclic Aromatic Hydrocarbons (PAHs). A number of organic chemicals are now being synthesized and they are so numerous and their toxicity values so profound that it is becoming a threat when released as hazardous wastes. The manufacturing of petrochemical compounds generate a variety of hazardous semi solid and heavy tarry residues known as back-end from the distillation columns in the plants (M. Ghaheri, and S. Ghaheri, 2007). Polycyclic aromatic hydrocarbons (PAHs) are compounds of environmental and human health concern since some PAHs have been shown to be toxic, mutagenic, and carcinogenic (Mrozik .A, et. al, 2003). Human exposure to PAHs may occur from emissions during the incomplete combustion of fossil fuels or due to accidental discharge into aquatic and terrestrial environments during the transport, use, and disposal of petroleum products (Esmaeil. S, et. al, 2000). The naphthalene present in petrochemical waste is taken as a representative toxic contaminant for bioremediation. Naphthalene, a dicyclic aromatic hydrocarbon, and its methylated derivatives are considered some of the most acutely toxic compounds in the water-soluble fraction of petroleum (Michael A. Heitkamp et. al, 1987). Exposure to naphthalene has caused a decrease in hemoglobin concentration and inhibited oxygen consumption in various experimental organisms. PAH metabolism in the human body produces epoxide compounds with mutagenic and carcinogenic properties, cases of lung, intestine, liver, pancreas and skin cancer being reported (Samanta et al, 2002) PAHs are widespread in the environment, being found in air, water and soil. They are formed naturally during the incomplete combustion of organic matter or by many anthropogenic activities, such as the petrochemical industry and oil refining. In these activities, PAHs are present in the effluent as complex mixtures of low bioavailability that are highly limiting for conventional remediation techniques (Rodrigo J.S. Jacques et. al. 2005).The adapted microorganisms at the contaminated site have the versatility for the biodegradation of PAHs. The majority of bioremediation techniques make use of these indigenous, resident microbes. However, careful understanding of many interrelated factors is essential to achieve bioremediation (Fulekar M.H, 2005).
The present study deals with the characterization of soil and isolation of potential naphthalene degrading bacteria from petrochemical contaminated soil samples collected from petroleum refining area.
Materials and Methods
The samples were collected from the waste disposal site of the petrochemical industry for characterization of contaminants for its physico-chemical and biological parameters and to identify potential microorganism capable of degrading naphthalene.
Soil sampling
The soil was collected from area around petrochemical industries and oil refineries at Wardha road, Khapri, located about 12 kms from Nagpur city. The effluent and petrochemical waste which contains a mixture of various PAH, motor oils and grease are being dumped at this site through underground channels since past 30 years. Soil samples were collected aseptically from a layer 0–30 cm deep site a few meters away from the petrochemical plant. The soil samples were collected in sterilized seal pack polythene bags which was later ground and sieved through a 2mm pore size sieve and stored at 4°C for physico-chemical characterization and microbial assay for isolation of potential PAH degrading microorganisms.
Media Used
Tanner’s Mineral Medium (MM) containing naphthalene as a sole source of carbon was used for isolation of Naphthalene degrading bacteria. Mineral medium used was composed of (g/L deionized water) 0.04 CaCl2.H2O; 0.1 KH2PO4; 0.8 NaCl; 1.0 NH4Cl; 0.2 MgSO4.7H2O; 0.1 KCl. Micronutrients used were (mg/L) 0.1 CoCl2.6H2O; 0.425 MnCl2 .4H2O; 0.05 ZnCl2; 0.015 CuSO4.5H2O; 0.01 NiCl2.6H2O; 0.01 Na2MoO4.2H2O; 0.01 Na2SeO4.2H2O. The pH was adjusted to 7.0 with either HCl or NaOH.
Physico-chemical analysis of sample
The soil/sediment samples were sieved and stored at room temperature for physico-chemical analysis. The parameters were analyzed as per the “APHA, Standard Methods for Water and Waste Water Analysis.” (APHA, 1989, 1979). The samples were analyzed for the various quality parameters like pH, bulk density, moisture content, acidity, alkalinity, dissolved oxygen (DO), Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Sulfate (S), Nitrogen (N), Phosphate (P), in the laboratory.
Microbial analysis
The sample was used for microbial enumeration immediately after collection. For isolation of bacteria from the petrochemical contaminated soil/sediments, 1 gm of the mixed soil was added to 9ml of deionized water and 0.1 ml of this diluted sample was spread plated on Nutrient Agar medium from the appropriate dilution tubes and incubated at room temperature for 24 hrs. Only the plates showing isolated colonies were tallied, and the results were determined for each soil samples. The fungal colonies were counted after 48-72 hrs of incubation. Isolated colonies were plated on specific agars which were used for identifying presence of E. coli, Salmonella, Shigella, Vibriocholerae, Yeast and Mould, S. aureus, Clostridium, Pseudomonas, Streptococcus faecalis, and Serratia in the contaminated soil.
Isolation and characterization of naphthalene degrading bacteria
Spiking of organic compound
Screw capped bottles were used for the experiment in order to prevent the evaporation of naphthalene during the course of the study. Tanner’s Mineral Medium and screw capped bottles were autoclaved at 121°C for 20 minutes. Autoclaved bottles were dried in oven. A 1 ml aliquot of acetone containing specific amount of naphthalene was aseptically added to the screw capped bottles allowing the acetone to evaporate. After complete evaporation of acetone from the bottles, 100 ml of Tanner’s Mineral Medium was added under laminar hood so as to reach the desired concentration of organic compound (Fulekar and Geetha, 2008).
For the isolation of naphthalene-degrading bacteria, soil samples (1 g) was diluted in 9ml deionized water aseptically of which 1 ml was added to nutrient broth and incubated for 24 h at 150 rpm at 30°C. 1ml of culture obtained on nutrient broth was added to 100 ml Tanner’s Mineral Medium in screw capped bottles, spiked with 25 mg/l naphthalene as a sole source of carbon. The medium containing mixed culture and naphthalene was incubated at 30°C with orbital shaking (150 rpm). A 1ml aliquot was transferred every 10 days to the same sterile medium and incubated under the same conditions. pH and optical density at 600 nm were monitored during the course of experiment to analyze bacterial growth. After 10 days of incubation, 1ml of bacterial culture was transferred to higher concentration of naphthalene viz. 50 mg/l. This was subsequently transferred to 75 mg/l, and 100mg/l to get potential microorganism capable of biodegrading naphthalene. Further, 1ml of culture from 100 mg/l spiked medium was diluted in saline solution, plated on nutrient agar (peptone, meat extract and agar) and incubated for 24 h at 30°C in dark. Bacterial colonies obtained were purified on the same medium.
The degradative phenotype isolated was assessed by spread plating the colonies onto minimal agar with naphthalene supplied in the covers of the inverted plates as the sole carbon and energy source. This was incubated for 10 to 14 days in at 30°C in dark.
Identification of isolated microorganism by 16SrRNA analysis
The 16S rDNA was PCR amplified using Universal Primers. DNA isolated from pure culture was used as template. PCR was performed with a 50μl reaction mixture containing primer 16S, DNA template buffer, MgCl2, dXTPs, Taq polymerase. PCR products were analyzed by electrophoresis in 1.8% agarose gel. PCR program was carried out in PTC-200 Peltier Thermocycler.
The sequence data was aligned and analyze on NCBI, BLAST database to identify the bacterium and its closest neighbors (Dipty and Fulekar, 2009). DNA sequences were compared with already submitted sequence in database using BLAST. Further, most similar sequences were aligned by ClustalW and ClustalX software and phylogenetic tree was draw to analyze evolutionary relationships among sequences of isolated microorganism and nearest neighbors.
Results and Discussion
The waste disposal site of petrochemical industry located at Khapri, near Nagpur, Maharashtra have been studied for the physico-chemical and biological characteristics. The physico-chemical parameters viz. pH, bulk density, moisture content, Acidity, Alkalinity, dissolved oxygen (DO), Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Sulfate (S), Nitrogen (N), Phosphate (P) of soil/sediments collected from these waste disposal area were studied. The concentration of each parameters obtained are presented in table-1. The pH was found ranging from 7.5 to 7.6 with an (average value: 7.6) which provides the alkaline conditions for growth and multiplication of microbial consortium. The average temperature was around 28°C which provided mesophilic conditions for microbial consortium to survive at contaminated site. The moisture content (12.38%) in the soil was sufficient for survival and growth of microorganisms. The nutrient content such as Sulphate (196.25 mg/l), Phosphate (20.55 mg/l) and Nitrogen (0.44%) in soil were studied. The values of these nutrients suggest that the microbes were using these nutrients and carbon from the organic contaminated site. Besides, the DO (3.12 mg/l), BOD (3.12 mg/l) and COD (211.65%) values also suggest that ambient conditions are present for growth and proliferation of microbial consortium at the petrochemical contaminated site. The microbial characterization of soil/sediments (Table-2) showed the presence of Pseudomonas aureginosa, Streptococcus fecalis and Salmonella in the petrochemical contaminated soil.
The microbial consortium was exposed to increasing concentration of naphthalene ranging from 25 mg/l upto 100 mg/l in MSM. The single microbial colony found surviving at higher concentration of naphthalene as a sole source of carbon was further identified by 16S rDNA analysis. The sequence and the phylogenetic tree (Figure-1 and 2; table-4) have been used as a basis for identification of potential microorganism responsible for bioremediation of organic compound with special reference to naphthalene. The biochemical tests (table-3) and the 16S rDNA further confirmed that the potential microorganism was Achromobacter insolitus. The research work carried out by (Hyung-Yeel Kahng et. al, 2005., Jong-Su Seo et. al., 2007) also reported biodegradation of organic compounds by Achromobacter sp. Our research findings agreed that it is a potential microorganism for bioremediation of naphthalene. The adapted microorganism Achromobacter insolitus can be used as a culture for microbial biodegradation of naphthalene which can remediate naphthalene in soil/water contaminated environment.
 |
Figure 1: 16S rDNA Sequence of Achromobacter insolitus-Sequence BI 357.
|
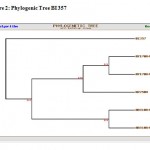 |
Figure 2: Phylogenic Tree BI 357.
Click here to View figure |
Table-1: Physical and chemical parameters of soil.
| Sr. no. | Parameters | Sample I | Sample II | Sample III | Sample IV | Average Value | |
| 1) | Temperature | 27°C | 29°C | 30°C | 27°C | 28°C | |
| 2) | Color | Brown | Brown | Brown | Brown | Brown | |
| 3) | Odor | Pungent | Pungent | Pungent | Pungent | Pungent | |
| 4) | pH | 7.51 | 7.64 | 7.63 | 7.63 | 7.60 | |
| 5) | Bulk density | 0.85 | 1.15 | 1.17 | 1.16 | 1.08 | |
| 6) | Moisture content (%) | 12.54 | 12.19 | 12.43 | 12.37 | 12.38 | |
| Chemical parameters of soil | |||||||
| 1) | Acidity (mg/L) | 54 | 64.23 | 58.5 | 65.66 | 60.59 | |
| 2) | Alkalinity (mg/L) | 700 | 706.66 | 590 | 486.66 | 620.83 | |
| 3) | Dissolved Oxygen (mg/L) | 4.5 | 1.7 | 3.56 | 2.7 | 3.12 | |
| 4) | Biological Oxygen Demand (mg/L) | 4.5 | 1.7 | 3.56 | 2.7 | 3.12 | |
| 5) | Chemical oxygen demand (mg/l) | 196 | 221.3 | 237.3 | 192 | 211.65 | |
| 6) | Phosphate (mg/L) | 21.4 | 30.2 | 10.9 | 19.7 | 20.55 | |
| 7) | Sulphate (mg/L) | 200 | 350 | 85 | 150 | 196.25 | |
| 8) | Total Organic carbon (%) | 3.6 | 3.2 | 4.2 | 2.8 | 3.45 | |
| 9) | Total organic Matter (%) | 6.21 | 5.17 | 7.24 | 4.83 | 5.86 | |
| 10) | Nitrogen (%) | 0.69 | 0.38 | 0.40 | 0.32 | 0.44 | |
Table 2: Microbial Characteristics of Soil
| Sr. No. |
Parameters
|
Test Method | Microbial range |
| 1. | Total Viable count/g | Nutrient Agar | 2500 |
| 2. | Total Coliform Count /g | Lauryl Tryptose Broth | 360 |
| 3. | E.Coli /25g | Eosin Methylene Blue Agar | Absent |
| 4. | Total Yeast and Mold count/g | Yeast and Mould Agar | 2100 |
| 5. | S.aureus /25g | Blood Agar | Absent |
| 6. | Clostridium/25g | Egg Yolk Agar | Absent |
| 7. | Vibriocholerae/25g | CBC Agar | Absent |
| 8. | Pseudomonas aureginosa/25g | Cetrimide Agar | Present |
| 9. | Streptococcus faecalis /25g | Bile Esculin Azide agar | Present |
| 10. | Salmonella /25g | Salmonella and Shigella Agar | Present |
| 11. | Shigella/25g | Salmonella and Shigella Agar | Absent |
Table 3: Biochemical characterization of Achromobacter insolitus:
| Test | Result | Test | Result |
| Lysine decarboxylase | – | Catalase | + |
| Ornithine decarboxylase | – | Oxidase test | + |
| Urease activity | – | Methyl red | – |
| Phenylalanine deaminase | – | Indole | – |
| Nitrate reduction | + | Malonate | – |
| Hydrogen sulfide production | – | Voges-proskauer’s | – |
| Citrate utilization | – | Glucose | – |
| Esculine | – | Arabinose | – |
| Xylose | – | Rhamnose | – |
| Cellobiose | – | Saccharose | – |
| Raffinose | – | Lactose | – |
Table 4: Hit List and Classification of the nearest neighbors
|
Sample BI 357 |
Gene Bank Entry | Domain | Phylum | Class | Order | Family | Genus | Species |
| AY170847 | Bacteria | Proteobacteria | Betaproteobacteri | Burkholderiales | Alcaligenaceae | Achromobacter | insolitus
|
|
| AY170848 | Bacteria | Proteobacteria | Betaproteobacteri | Burkholderiales | Alcaligenaceae | Achromobacter | spanius
|
|
| AB010840 | Bacteria | Proteobacteria | Betaproteobacteri | Burkholderiales | Alcaligenaceae | Achromobacter | ruhlandii | |
| AB010840 | Bacteria | Proteobacteria | Betaproteobacteri | Burkholderiales | Alcaligenaceae | Achromobacter | piechaudii
|
|
| M22509 | Bacteria | Proteobacteria | Betaproteobacteri | Burkholderiales | Alcaligenaceae | Achromobacter | xylosoxidans
|
Conclusion
The petrochemical waste characterized for physico-chemical and biological parameters including microbial consortium present in the contaminated site were studied to identify the potential microorganism capable of biodegrading the organic pollutant with special reference to naphthalene. The identified microbe “Achromobacter insolitus” would serve as potential microorganism for biodegradation of organic pollutant.
References
- Ghaheri .M, Ghaheri .S, “Hazardous Sludge wastes of Petrochemical Industries in the Developing Countries”. Environmental department of Abadan Petrochemical Company (APC), (2007).
- Mrozik .A, Piotrowska-Seget .Z, Labuzek .S. “Bacterial Degradation and Bioremediation of Polycyclic Aromatic Hydrocarbons.” Polish Journal of Environmental Studies, Vol. 12, No. 1, 15-25, (2003).
- Esmaeil. S., Alsaleh and Jermy R. Mason., “In situ bioremediation of polynuclear hydrocarbons in gas manufacturing sites, possible role of indigenous and augmented bacteria.” 1st edn. Division of Life Science, Kings College, London, Campeden, Hill road, W8 7AH, U.K.(2000).
- Michael A. Heitkamp, James P. Freeman, and Carl E. Cerniglia. “Naphthalene Biodegradation in Environmental Microcosms: Estimates of Degradation Rates and Characterization of Metabolites”, National Center for Toxicological Research, Food and Drug Administration, Jefferson, Arkansas 72079, Applied Environmental Microbiology, Vol. 53, p. 129-136 (1987).
- Samanta, S. K., O. V. Singh, and R. K. Jain. “Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation.” Trends in Biotechnology. 20:243-248 (2002).
- Rodrigo J.S. Jacquesa, Eder C. Santosa, Fa´ tima M. Bentoa, Maria C.R. Peralbab, Pedro A. Selbacha, Enilson L.S. Sa´ a, Fla´ vio A.O. Camargoa., “Anthracene biodegradation by Pseudomonas sp. isolated from a petrochemical sludge landfarming site”. International Biodeterioration & Biodegradation., Vol. 56 (2005).
- Fulekar M. H. “Environmental Biotechnology.” Oxford and IBH publishing house, New Delhi, India. 74-75 (2005)
- APHA, Standard methods for examination of water and waste water. American Public Health Association, New York (1979).
- APHA, Standard methods for examination of water and waste water. American Public Health Association, New York (1989).
- Fulekar M. H. and Geetha M., “Bioremediation of chlorpyrifos by Pseudomonas aeruginosa using scale up technique.” Journal of Applied Biosciences.12:657-660 (2008)
- Dipty Singh and Fulekar M. H. “Bioremediation of phenol by a novel partitioning bioreactor using cow dung microbial consortia” Biotechnology Journal, wiley inter science (March 2009)
- Hyung-Yeel Kahng and Kye-Heon Oh. “Molecular Detection of Catabolic Genes for Polycyclic Aromatic Hydrocarbons in the Reed Rhizosphere of Sunchon Bay.” Journal of Microbiology. Vol 43 (2005).
- Jong-Su Seo, Young-Soo Keum, Renee M. Harada, and Qing X. Li., “Isolation and Characterization of Bacteria Capable of Degrading Polycyclic Aromatic Hydrocarbons (PAHs) and Organophosphorus Pesticides from PAH-Contaminated Soil in Hilo, Hawaii.”Journal of. Agricultural and Food Chemistry. 55 (14), 5383-5389 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.





