How to Cite | Publication History | PlumX Article Matrix
J. P. Mairiga* and P. G. C. Odeigahh
Department of Cell Biology and Genetics, University of Lagos, Akoka Nigeria.
ABSTRACT: A cinical study of Isoniazid (INH) acetylator phenotype was carried out in 79 in-patients under going pulmonary Mycobacterium tuberculosis treatment in Alushi Medical Center in Nasarawa State. Isoniazid acetylator in the tuberculosis patients was determined using dosage and urinalysis technique. The distribution was bimodal with an antimode at 70-74%. On this basis, Forty (40) or 50.63% of the sampled population of the patients were rapid acetylators, thirty six (36) or 45.57% were slow acetylators, while three (3) or 3.80% were unclassified. The calculation of gene frequencies using Hard Weinberg theorem showed a dominant allelic (R) Frequencies of 0.2974 and 0.7026 for recessive allele. Inferences drawn suggest that a high proportion of the rapid acetylators from the sampled population may be heterozygous.
KEYWORDS: Acetylator; phenotype; tuberculosis; isoniazid; urinalysis
Download this article as:| Copy the following to cite this article: Mairiga J. P, Odeigahh P. G. C. Clinical Assessment of Isoniazid (INH) Acetylator Phenotype of Mycobacterium Tuberclulosis Infected Patients in Alushi-Akwanga. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Mairiga J. P, Odeigahh P. G. C. Clinical Assessment of Isoniazid (INH) Acetylator Phenotype of Mycobacterium Tuberclulosis Infected Patients in Alushi-Akwanga. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=7860 |
Introduction
Isoniazid (INH) other wise called Isonicotinyl hydrazide is an odorless white crystalline powder which is freely soluble in water and has been considered the most powerful drug used in the initial treatment of tuberculosis in both developing and industrial nations (Robert et al, 1996, Neil, 2005, Ramachandran et al, 2005).
Isoniazid is administered orally and is metabolized mainly by acetylation or inactivation in the Liver by the enzyme N-acetyltransferase. Though, the capacity to metabolize INH varies greatly in Mammals, it has been reported to differ among human but constants in each individual (Mancinelli et al, 2000).
The degree of inactivation of INH is measured by examining the level of free INH in the blood serum or urine at various times after ingestion of certain dose. Accordingly, patients are classified into two distinct groups ‘Rapid’ and ‘Slow’ acetylators or in activators respectively on the basis of the level and manner in which they metabolized the drug (Burroughs et al, 2002).
Whereas the slow acetylator phenotype is due to homozygosity for an autosomal recessive gene, the rapid acetylator is due either to homozygosity or heterozygosity for an autosomal gene, implying that the acetylation rate of isoniazid could be hereditary and mainly the results of two alleles (Ramachandran et al, 2005).
Information on the rate of acetylator phenotype among indigenous Nigerian population is not much. However, earlier studies involving Nigerian population were conducted by Fawcet and Gammon (1975) who published a 51% rapid acetylator phenotype in a Northern Nigerian subjects, while Salako et al, (1977), reported a 60% rapid acetylation in their study of the rate of isoniazid acetylation in 220 Nigerians mainly of Yoruba ethic group in Ibadan. Other investigations include Afonja, et al, (1979) and Odeigah et al, (1989) who posited a 57% and 62% rapid acetylation of the sampled populations respectively.
Our interest in the metabolism of Isoniazid is informed by the findings that in some certain individuals, the anti tuberculosis drug INH disappears more quickly from the blood than in others and those referred to as slow acetylators tend to show more side effects, while the rapid acetylators respond poorly to the regime due to fast clearing ability.
The implication is that, patients who acetylate Isoniazid at slow rate have high chances of developing side effects more often and higher frequency of reversal of infectiousness than those who acetylate fast. This is because patients who clear drug rapidly from the body would always require more of the drug to attain therapeutic effect than the patients of low clearing capacity. Similarly, drug accumulation resulting from drug administration could produce toxicity in patients with slow clearing ability (Odeigah et al, 1989).
Most importantly is the fact that health resources are limited in most countries and localities, hence, the choice of regime is largely determined by the cost of drugs, their accessibility and the organizational capacity of the health services. These constrains have resulted in constant changes in the regimes of tuberculosis, important among is the shift to ambulatory treatment and short course chemotherapy using a combination of Isoniazid and other drugs such as Rifampicin, Pyrazinamide, Ethambutol, Thiocetazone and Streptomycin (Orjioke et al, 1997)
Mycobaterium tuberculosis is a prototype of the slow acid growing, acid fast mycobacterium, whose ‘Host’ is the Human (Mark, 1993) WHO estimates that about 16-20 million are recorded World wide and in Nigeria, it is estimated that about 100,000 sputum positive cases are recorded yearly (Neil, 2005). The probability of infection depends on the intensity of exposure and the effectiveness of innate host defenses (Robert et al 1996). Indeed, the spread of tuberculosis is one of the more present crises currently facing the human global community.
Study on Isoniazid acetylator phenotype of mycobacterium tuberculosis infected population in the Middle Belt of Nigeria is limited. This study on the Clinical assessment is therefore necessary as a proviso for the possibility of new anti-tuberculosis drug dosage regimen for patients suffering from tuberculosis and in overseeing the chemotherapeutic effects of the drug, through the study of phenotypic frequencies of the rapid and slow acetylators in the population.
Materials and Method.
Participants
The subjects were Nigerians from three States namely, Nasarawa, Plateau, and Benue, states respectively. The sampled population comprised of twelve tribes (Hausa, Migilli, Eggon, Rindere, Mada, Tiv, Kanuri, Angas, Alago, Bassa, Agatu, and Nimzo). Indigenes of these states constitute part of the Middle Belt of Nigeria.
A total of 79 (45 Males and 34 Females) in patients suffering from pulmonary tuberculosis in Alushi Medical Center (TB/Leprosy referral center) Akwanga, Nasarawa State Nigeria, ranging from 15-54 years were used.
Conduct of the study
The subjects were served 300mg of Isoniazid after breakfast in single dose according to their body weight and under the strict supervision of the Medical staff. Some patients in addition to Isoniazid (INH) had Rifampicin (150mg), Pyrazinamide (400mg), Ethambutol (400mg), Thiocetazone (350g), and 0.75g of Streptomycin. At approximately 8 hours later, the subjects were asked to collect sample of their urine into a labeled specimen container. The samples were analyzed within 24 hours of collection.
Urinalysis
Firstly, each urine specimen was tested for sugar and a red color change indicated the presence of Sugar, hence it was discarded, since urine of the diabetic gave false positive reaction with Isoniazid (Mason and Russel, 1971).
This was followed by analytical method, a modification of Eidus et al, (1973) which was based on the estimation of the proportion of acetylisoniazid excreted in urine. The optical densities (OD) of the two aliquots were then measured at a wave length of 550 nm.
Determination of Inh Acetylation Status
Considering that aliquot A. contained only acetylisoniazid while aliquot B contained both acetylisoniazid and free isoniazid converted to acetylisoniazid, the percentage of acetylation was obtained thus: Optical Density of A / Optical Density of B X 100. By these an individual was classified either as Rapid or Slow acetylator based on the percentage of acetylation.
Results
The acetylator phenotype of the overall population sampled is presented in a histogram. The histogram in Figure 1 shows a bimodal distribution, separating the two classes of acetylators into rapid and slow at an antimode of 70-74%. The phenotype was assigned by defining the rapid acetylator as one whose percentage Isoniazid acetylation is greater than 74% as against slow acetylators whose percentage Isoniazid acetylation is less than 70%. Based on this categorization, 40 (50.63%) of the subjects were rapid acetyators while 36 (45.57%) were slow acetylators.
The frequencies of rapid and slow acetylators among the sexes indicated that 21 Male representing 46.67% were rapid acetylators, while 24 representing 53.33% were slow acetylators. Similarly, 19 and 15 females representing 55.88% and 44.13% were rapid and slow acetylators respectively (Table 1). A high incidence of slow acetylator phenotype was detected among the Eggon ethnic group, with a 60% value compared to the Mada and Hausas who had 30 and 36.37% respectively (Table 2). Among the sampled age groups, 41 representing 51.89% were slow acetylators, as against 38 being 48.10% (Table 3).
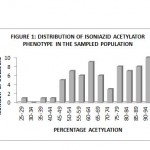 |
Figure 1:
|
Table 1: Distribution of acetylator phenotype according to sexes.
| SEX | RAPID
ACETYLATOR |
PERCENTAGE OF RAPID ACETYLATOR | SLOW ACETYLATOR | PERCENTAGE OF SLOW ACETYLATOR |
| MALE
|
21 | 26.58 | 24 | 30.38 |
| FEMALE
|
19 | 24.10 | 15 | 18.99 |
| TOTAL
|
40 | 50.68 | 39 | 49.37 |
Table 2: Distribution of INH acetylation among three major ethnic groups.
| Major groups | Rapid acetylators | Percentageof rapid acetylators | Slow acetylators | Percentage of slow acetylators.
|
| Hausa
|
7 | 63.67 | 4 | 36.37 |
| Eggon
|
6 | 40.00 | 9 | 60.00 |
| Mada
|
9 | 69.23 | 4 | 30.77 |
Table 3. Distribution of INH acetylation among the age groups.
| AGE GROUP
(YEARS) |
RAPID ACETYLATORS | PERCENTAGE OF RAPID ACETYLATORS | SLOW ACETYLATORS | PERCENTAGE OF SLOW ACETYLATORS |
| 15-19 | 10 | 12.66 | 3 | 3.80 |
| 20-24 | 6 | 7.36 | 8 | 10.13 |
| 25-29 | 9 | 11.39 | 9 | 11.39 |
| 30-34 | 9 | 11.39 | 8 | 10.13 |
| 35-39 | 0 | 0.00 | 4 | 5.06 |
| 40-44 | 3 | 3.80 | 6 | 7.36 |
| 45-49 | 0 | 0.00 | 1 | 1.27 |
| 50-54 | 1 | 1.27 | 2 | 2.53 |
| TOTAL | 38 | 48.10 | 41 | 51.89 |
The genotypic frequencies expected in the population under Panmixis were determined using the Binomial Expression p2 + 2pq + q2 = 1. In which the frequencies of the homozygous rapid acetylators (RR), heterozygous rapid acetylators (Rr) and homozygous slow acetylators (rr) were found to be 8.84%, 41.79%, and 49.37% respectively.
Hardy-Weinberg theorem was used to calculate the gene frequencies of both alleles with the assumption that the sample of the population used is a representation of the population in equilibrium, resulting in a 0.2974 frequency of dominant allele and 0.7026. of recessive allele. The calculation of Chi square less than two degrees of freedom were statistically significant at 0.00000125.
Inferences drawn from these results suggest that a high proportion of rapid acetylators from the population sampled may be heterozygous.
Discussion
Numerous studies have demonstrated that differences exist in Isoniazid desposition or elimination and are attributable to genetically determined differences in individual N- acetylation capacity (Weber, et al, 1985). Also that the outcome of intermittent Isoniazid chemotherapy could be related to the individual patient’s acetylator phenotype, this shows that the metabolism of Isoniazid in man is polymorphic.
In their study, Fawcet and Gammon (1975) reported 51% rapid acetylator and 49% slow acetylators on Isoniazid phenotyping of 109 non tuberculosis patients in Northern Nigeria, further investigation by Ellard and Gammon, on Isoniazid phenotyping revealed that 45% of their East African tuberculosis patients are rapid acetylators, Salako and Aerounmu (1977) in their study of 220 southern made comprising mainly Yoruba and Ibos, found that 60 and 40% were rapid and slow acetylators respectively. In another investigation of 198 Southern Nigerian populations, Afonja et al 1979, reported that 55% were rapid acetylators and 45% slow acetylators. Odeigah and Okonuwo in 1989 found that a 61.92% of the population sampled for Isoniazid acetylation were rapid acetylators.
The findings on the Clinical assessment of Isoniazid acetylator phenotype and response to treatment showed that the rate of acetylation of Isoniazid had no appreciable influence on the response of patients to treatment including INH on daily regime, since both rapid and slow acetylators showed no marked differences in their mode of acetylation. This finding compares favorably with the findings of the previous investigators on Indigenous Nigerian population. The only differences between this finding and the other investigator’s might be due to the limited number of the sampled population for the study and likely, the criteria used in delimiting the phenotypes into rapid and slow.
However In this investigation, it was also deduced that age has no significant influence on the rate of Isoniazid acetylation, considering the similarity in the trends of the frequencies across the age groups. This may be contrary to the expectation that age has some influence on the acetylation phenotype since children are known to have prolong half life than adults (Miceli et al, 1981) Similarly findings on ethnic groups contains insufficient number of tuberculosis subjects and limited representation of other ethnic groups to show whether or not there is a significant association in their acetylation status in relation to tuberculosis infection, even though a consideration of three tribes: Hausa, Eggon and Mada, indicates that the Hausas and Mada subjects are rapid acetylators ( 63.67 and 69.23% respectively) while the Eggons are slow acetylators with 60.00% acetylation status.
The implication of these findings is that the population studied might respond well to the combined formulation of chemotherapeutic regime given to pulmonary tuberculosis patients on daily basis. Further investigation is necessary on the possibility for genetic variability of anti tuberculosis drug response and dosage regimes, to help address the issue of resistant bacilli and the polymorphic acetylation status of individuals.
References
- Afonja, A. O, Arhavwarien E. D, Okotore R. O, and Femi P.D. Isoniazid Acetylation Phenotype of Nigerians. Nigerian Medical Journal (1979); 9:86-88.
- Burroughs V.J, Maxey R. W, Levy R.A Racial and Ethnic Differences in Response to Medicines; Towards Individualized Pharmaceutical Treatment. Journal of the National Medical Association (2002) Vol. 94(10)1-25.
- Eldus L, Varughese P. Hodgkin M.M. and McRae K.B Simplification of Isoniazid Phenotyping procedure to promote the application is the Chemotherapy of tuberculosis. WHO (1997); 49:507-5.
- Fawcet I.W, and Gammon P.T. Determination of the acetylator phenotype in a Nothern Nigeria population. Tubercule (1975); 56: 199.
- Ramachandran G, Kumar A.K.H, Rajasekaran S, Padmanpyriyadarsini C, Swaminathan S, Vankatesan P, Sekar L, Gurumurthy PAcetylator Status influences Bioavailability of Isoniazid in patients with Advanced HIV disease. SAARC JTBLDIS and HIV/AIDS, (2005); 11(2)9-12.
- Orjioke C. J, Sofola T.O, Chitimba N, Verduin P.R.N, National Tuberculosis and Leprosy control prog. Worker’s manual(1997); 184p.
- Mancinelli L, Cronin M, Sadee W, Pharmacogenomics: The promise of personalized Medicine. The Pharmsc. Journal(2002); 2(1).
- Miceli J.W, Olson W.A, Cohen S.N, Elimination Kinetics of Isoniazid in the New born infant. Developmental Pharmacology and Therapeutics(1981); 2: 235-239.
- Neil W.SThe Pathogenesis of tuberculosis: The first hundred and twenty three years. American Journal of Respiratory cell and Molecular Biology (2005); Vol. 32; 251-256.
- Robert V, Israili Z.H, AND Pruit A. W In Vitro absorption studies of INH. Human Experimental Toxicology . (1996); 10:133-135.
- Odeigah P.G.C, and Okunowo M.A High Freqquency of the rapid Isoniazid acetylator phenotype in Lagos. Human Genetics(1989); 39; 26-31.
- Salako L.A, and Aderounmu F.A Determination of the Isoniazid acetylator phenotype in a West African Population. Tubercle (1977); 58: 109-112.
- Marks G.L Genetics of Tuberculosis. Medical Clinics of North Ameriica (1993); 1219-1234.
- Mason E. and Russel D.W Determination of the Isoniazid acetylation rates ( phenotypes) of patients being treated for tuberculosis. Bull. WHO (1971); 45; 617-624.
- Weber W.W, and Hein D.W, N-acetylation Pharmocogenetics. Pharmacological Review (1985 ) 37:26-79.

This work is licensed under a Creative Commons Attribution 4.0 International License.





