How to Cite | Publication History | PlumX Article Matrix
V. Suseela1*, V. K. Gopalakrishnan1 and D. Chandrapraba2
1Department of Biochemistry, Karpagam University, Coimbatore - 641 021 India. 2Department of Biochemistry, Sree Narayanaya Guru college, Coimbatore - 641 021 India. Corresponding Author E-mail: suseelavivekananthan@yahoo.com
ABSTRACT: Antioxidant potential of leaves of Artemisia nilagirica was studied using different in vitro models like 1,1-diphenyl-2-picryl hydrazyl (DPPH), 2,2-azinobis-(3-ethylbenzothizoline-6-sulphonate) (ABTS), nitric oxide, superoxide, hydroxyl radical and lipid peroxidation. The ethanol extract showed maximum scavenging activity (89.06%) of ABTS radical followed by the scavenging of DPPH radical (84.94%) and superoxide radical (79.69%) respectively. Total antioxidant capacity was found to be 61.3 mg ascorbic acid equivalents at 500μg/ml extract concentration. However, the extract showed only moderate lipid peroxidation inhibition activity. Total phenols and flavonoids were found to be 69.71 + 1.7 mg Gallic acid equivalents/g of dry material and 28.41 + 0.6 mg Quercetin equivalents/g of dry material respectively. These results suggest that phenolics and flavonoids in the leaves provide substantial antioxidant activity.
KEYWORDS: Artemisia nilagirica; free radical; in vitro studies
Download this article as:| Copy the following to cite this article: Suseela V, Gopalakrishnan V. K, Chandrapraba D. Evaluation of Free Radical Scavenging Potential of Ethanolic Extract of Artemisia Nilagirica (Clarke) Pamp. Leaves. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Suseela V, Gopalakrishnan V. K, Chandrapraba D. Evaluation of Free Radical Scavenging Potential of Ethanolic Extract of Artemisia Nilagirica (Clarke) Pamp. Leaves. Biosci Biotechnol Res Asia 2008;6(1). Available from: https://www.biotech-asia.org/?p=8093 |
Introduction
Free radicals have been implicated in causation of ailments such as liver cirrhosis, atherosclerosis, cancer, diabetes etc.1 Reactive oxygen species (ROS) such as superoxide anions (O2–), hydroxyl radical (OH.) and nitric oxide (NO) inactivate enzymes and damage important cellular components causing injury through covalent binding and lipid peroxidation2. Antioxidants may offer resistance against the oxidative stress by scavenging the free radicals, inhibiting the lipid peroxidation and by other mechanisms and thus prevent disease3. Although a living system possesses several natural defense mechanisms, such as enzymes and antioxidant nutrients, which arrest the chain reaction of ROS initiation and production4, its continuous exposure for a long time may lead to irreversible oxidative damage. Many plants often contain substantial amounts of antioxidants including vitamin C and E, carotenoids, flavonoids and tannins etc., and thus can be utilized to scavenge the excess free radicals from human body5.
Artemisia nilagirica belongs to Asteraceae contains artemisimin essential oil and flavonoids. The extract of the herb is effective in curing malaria6. Leaves and flowering tops are bitter, acrid, thermogenic, aromatic, anodyne, anti-inflammatory, depurative, diuretic, emmenagogue, aphrodisiac, appetizer, digestive, stomachic, anthelmintic, febrifuge, deobstruent, alexeteric and haematinic. The plant is useful in vitated conditions of vata and kapha, cough, asthma, bronchitis, cephalagia, nervous and spasmodic affections, inflammations, leprosy, skin disorders, strangury, ammenorrhoea, dysmenorrhoea, anorexia, dyspepsia, flatulence, colic, intestinal worms, fever, hysteria, measles and anemia7. It is reported that the flowering tops of Artemisia nilagirica were effective in the muscle phase of T. spiralis infection8.
Materials and Methods
Materials-All chemicals and solvents used were of analytical grade; 2,2-azinobis 3-ethylbenzo-thiozoline-6-sulphonate (ABTS) was obtained from Sigma Chemicals, USA. The other chemicals used were 2-Thiobarbituric acid, mannitol, 2-deoxyribose, ferrozine, ferrous chloride, vitamin C, Folin-Ciocalteu reagent, 2,4-dinitrophenyl hydrazine, ferric chloride, potassium ferricyanide, hydrogen peroxide, aluminum chloride, sodium carbonate, sodium nitrite, ammonium persulphate, sodium hydroxide and ethanol were obtained from HiMedia Chemicals, Mumbai, India.
Plant material-The leaves of Artemisia nilagirica were collected in Coimbatore, Tamil Nadu. The plant material was identified and confirmed at Botanical Survey of India, Southern circle, Coimbatore and the voucher specimen (No. BPG 09) was retained in our laboratory for future reference.
Preparation of the plant extract- About 50g of air dried leaves were dissolved in 250ml of ethanol and kept in an orbital shaker for overnight. The obtained extracts were filtered with Whatman no. 1 filter paper and the filterate was collected. The ethanol was then removed under reduced pressure at 500C to yield (10.53%) a concentrated extract.
DPPH radical scavenging effect
In vitro DPPH radical scavenging activity was measured by the spectrophotometric method9. To a methanolic solution of DPPH (200µM), 0.05 ml of the test compounds dissolved in ethanol were added at different concentration (100-500 µg/ml). An equal amount of ethanol was added to the control. After 20 min, the decrease in the absorbance of test mixture (due to quenching of DPPH free radicals) was read at 517 nm and the percentage inhibition calculated by using the formula(1).
(Control-test)
Inhibition (%) = ___________ x 100 ___________ (1)
Control
Reducing power assay
The reducing power of the ethanol extract was carried out spectrophotometrically10. About 2.5 ml of different concentrations of the plant extract (100-500mg/ml), were mixed with 2.5 ml phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min, then rapidly cooled, mixed with 2.5 ml of 10% trichloroacetic acid and centrifuged at 3000 rpm for 10 minutes. About 2.5 ml of the supernatant was taken and 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride were added to it, mixed well and allowed to stand for 10 minutes. The absorbance was measured at 700 nm.
ABTS radical cation decolorisation assay
In this improved version11, ABTS.-, the oxidant is generated by persulfate oxidation of 2, 2-azinobis (3-ethylbenzoline-6-sulfonic acid)-(ABTS2-).
ABTS radical cation (ABTS+) was produced by reacting ABTS solution (7mM) with 2.45 mM ammonium persulphate and the mixture were allowed to stands in dark at room temperature for 12-16 hr before use. For the study, different concentration (100-500µg/ml) of ethanoloic extract (0.5 ml) were added to 0.3 ml of ABTS solution and the final volume was made up with ethanol to make 1ml. the absorbance was read at 745 nm and the percentage inhibition was calculated.
Nitric oxide radical Scavenging effect
Nitric oxide was generated from sodium nitroprusside and measured by Griess reaction12, 13. Sodium nitroprusside (5mM) in standard phosphate buffer solution was incubated with different concentration (100-500µg/ml) of ethanolic extract dissolved in phosphate buffer (0.025 M, pH 7.4) and the tubes were incubated at 250C for 5 hr. Control experiments without the test compounds, but with equivalent amounts of buffer were conducted in an identical manner. After 5 hr, 0.5 ml of Griess reagent (1% sulphanilamide, 2% O-phosphoric acid and 0.1% napthylethylene diamine dihydrochloride). The absorbance of the chromophore formed during diazotization of nitrite with sulphanilamide and its subsequent coupling with nathylethylene diamine was read at 546 nm.
Superoxide anion radical scavenging effect
The scavenging activity towards the superoxide radical (O2.-) was measured in terms of inhibition of generation of O2. 14. The reaction mixture consisted of phosphate buffer (50 mM, pH 7.6), riboflavin (20ug/ml), EDTA (12mM), NBT (0.1mg/3ml) and sodium cyanide (3ug/0.2 ml). Test compounds of various concentrations of the extract 100-500 µg/ml were added to make a total volume of 3ml. The absorbance was read at 530 nm before and after illumination under UV lamp for 15 min against a control instead of sample. The percentage inhibition was calculated by using the same formula as given above.
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity was measured according to the method of Kunchandy and Rao15 by studying the competition between deoxyribose and test extract for hydroxyl radical generated by Fenton’s reaction. The reaction mixture contained deoxy ribose (2.8 mM), FeCl3 (0.1 mM), EDTA (0.1 mM), H2O2 (1 mM), ascorbate (0.1 mM), KH2PO4- KOH buffer (20 mM, pH 7.4) and various concentrations of the sample extracts in a final volume of 1.0 ml. The reaction mixture was incubated for 1 h at 370C. Deoxyribose degradation was measured as thiobarbituric acid reacting substances (TBARS) and the percentage inhibition was calculated.
In vitro anti-lipid peroxidation assay
Freshly excised goat liver was processed to get 10% homogenate in cold phosphate buffered saline, pH 7.4 using glass Teflon homogenizer and filtered to get a clear homogenate. The degree of lipid peroxidation was assayed by estimating the TBARS by using the standard method16 with minor modifications. Different concentrations of the extracts (100-500 µg/ml) in water were added to the liver homogenate. Lipid peroxidation was initiated by adding 100 µl of 15mM ferrous sulphate solution to 3 ml of the tissue homogenate. After 30 Min, 100 µl of this reaction mixture was taken in a tube containing 1.5 ml of 10% TCA. After 10 min, tubes were centrifuged and supernatant was separated and mixed with 1.5 ml of 0.67% TBA in 50% acetic acid. The mixture was heated for 30 min in a boiling water bath. The intensity of the pink coloured complex was measured at 535 nm. The results were expressed as percentage inhibition using the formula as given above.
Determination of total antioxidant capacity
The method described by Prieto et. al.,17 was used to determine the total antioxidant capacity of the extract. The tubes containing 0.2 ml of the extract (100-500mg/ml), 1.8 ml of distilled water and 2 ml of phosphomolybdenum reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) were incubated at 95°C for 90 minutes. After the mixture had cooled to room temperature, the absorbance of each solution was measured at 695 nm using an UV/VIS spectrophotometer (Beckman DU-530).The antioxidant capacity was expressed as ascorbic acid equivalent (AAE).
Quantitative analysis of antioxidative components
Total phenolics (TPC)
Total phenolics were quantified and expressed as Gallic acid equivalents according to a method proposed by Singleton et. al.,18 . About 3.9 ml of distilled water and 0.5 ml of Folin-ciocalteau reagent were added to 0.1 ml of the extract in a tube and incubated at room temperature for 3 min, 2 ml of 20% sodium carbonate was added to this and kept at boiling water bath for 1 min. The blue colour formed was read at 650nm.
Total flavonoids (TFC)
TFC was estimated colorimetrically based on the method modified by Zhishen et. al.,19. To 0.1 ml of the ethanol extract in a 10 ml volumetric flask, distilled water was added to make the volume to 5 ml and 0.3 ml of 5% NaNO2 was added to this. About 3 ml of 10% AlCl3 was added 5 min later and after 6 min, 2 ml of 1 M NaOH was added to it. The absorbance was measured at 510 nm, Quercetin (50-250 µg/ml) was used as a standard for constructing a calibration curve.
Statistical analysis
Linear regression analysis was used to calculate IC50 values.
Results
Several concentrations ranging from 100-500µg/ml of the ethanolic extract of A. nilagirica were tested for their antioxidant activity in different in vitro models. It was observed that free radicals were scavenged by the test compounds in a concentration dependent manner in all the models. The percentage inhibition in various models viz., DPPH, ABTS, superoxide radical, nitric oxide, hydroxyl radical, lipid peroxidation is shown in Fig. 1-4. The IC50 values were found to be 70, 280, 295, 315, 245, 435µg/ml respectively. The reducing power of the extract was observed to be dose dependent as shown in Fig. 5
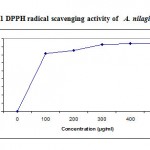 |
Figure 1: DPPH radical scavenging activity of A. nilagirica.
|
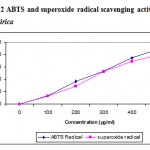 |
Figure 2: ABTS and superoxide radical scavenging activity of A. nilagirica.
|
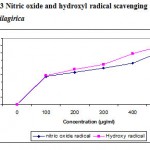 |
Figure 3: Nitric oxide and hydroxyl radical scavenging activity of A. nilagirica.
|
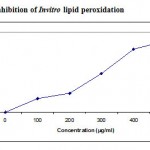 |
Figure 4: Inhibition of Invitro lipid peroxidation.
|
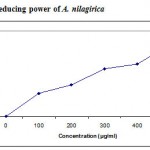 |
Figure 5: Reducing power of A. nilagirica.
|
Fig 6 illustrates the antioxidative capacities of various concentrations of A. nilagirica (100-500µg/ml). Total antioxidant capacity of the extract was found to be 62.3 mg ascorbic acid equivalents at 500µg/ml extract concentration. This good antioxidant activity might be attributed to the presence of phytochemicals, such as flavonoids and biflavones. Total phenolic content in A. nilagirica was found to be 69.71 + 1.7 mg Gallic acid equivalents/g of dry material. Total flavonoids in the extract were found to be 28.41 + 0.6 mg Quercetin equivalents/g of dry material.
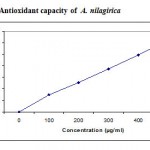 |
Figure 6: Antioxidant capacity of A. nilagirica.
|
Discussion
Free radicals are chemical entities that can exist separately with one or more unpaired electrons. The generation of free radicals can bring about thousands of reactions and thus cause extensive tissue damage. Lipids, proteins and DNA are all susceptible to attack by free radicals20.
The 1, 1-diphenyl 2-picryl hydrazyl (DPPH) radical was widely used as the model system to investigate the scavenging activities of several natural compounds such as phenolic and anthocyanins or crude mixtures such as ethanol extract of plants. DPPH is a relatively stable free radical and the assay determines the ability of ethanolic extract of A. nilagirica to reduce DPPH radical to the corresponding hydrazine by converting the unpaired electrons to paired ones, which in fact is the action of the antioxidants. The dose dependent inhibition of DPPH radical (Fig.1) indicates the ethanolic extract of A. nilagirica causes reduction of DPPH radical in a stoichometric manner14, 21.
ABTS assay is a decolourizing assay, which involves the direct generation of ABTS radical into monocation, which has a long wavelength absorption spectrum without involvement of any intermediary radical. The antioxidant activity of the extract by this assay implies that the action may be either inhibiting or scavenging radicals since both inhibition and scavenging properties of antioxidant towards this radical have been reported in earlier studies22.
The hydroxyl radical scavenging activity is measured as the percentage inhibition of hydroxyl radicals generated in the Fenton’s reaction mixture29 by studying the competition between deoxyribose and the extract for hydrogen radicals generated from Fe+/ascorbate/EDTA/H2O2 systems. The hydroxyl radicals attack deoxyribose which eventually results in TBARS formation. From the present results, it is observed that the extract of A. nilagirica have better hydroxyl radical scavenging activity as reflected in terms of percentage inhibition.
Nitric oxide is an important chemical mediator generated by endothelial cells, macrophages, neurons etc and is involved in the regulation of various physiological processes23. Excess concentration of NO is associated with several diseases24, 25. Oxygen reacts with the excess nitric oxide to generate nitrite and peroxynitrite anions, which acts as free radicals20, 26. In the present study the extract competes with oxygen to react with nitric oxide and thus inhibits the generation of the anions.
Superoxides are produced from molecular oxygen due to oxidative enzymes of body as well via non enzymatic reaction such as autooxidation by catecholamines31 . The scavenging activity towards the superoxide radical (O2.-) is measured in terms of inhibition of generation of O2.-. In the present study, superoxide radical reduces NBT to a blue colored Formosan that is measured at 560nm32. The result shows that the ethanolic extract of A. nilagirica has a potent scavenging activity with increasing percentage inhibition. The probable mechanism of scavenging the superoxide anions may be due to inhibitory effect of the extract towards generation of superoxides in the invitro reaction mixture.
The peroxidation of membrane lipids initiated by oxygen radicals may lead to cell injury. Initiation of lipid peroxidation by ferrous sulphate takes place through ferryl-perferryl complex27 or through .OH radical by Fenton’s reactions28 , thereby initiating a cascade of oxidative reactions. The results obtained in the present study may be attributed to several reasons viz., the inhibition of ferryl-perferryl complex formation; scavenging of .OH or superoxide radical or by changing the ratio of Fe3+//Fe2+; reducing the rate of conversions of ferrous to ferric or by chelation of the iron itself29.
Fe3+-Fe2+ transformation was investigated in the presence of samples for the measurements of the reductive ability. The reducing power of ethanolic extract ranged from 0.19 + 0.065 to 0.59 + 0.043 abs for 100ug/ml to 500µg/ml of extract. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity30.
Phosphomolybdenum assay used to determine the total antioxidant capacity was based on the reduction of MO (VI) to MO (V) by the extract and subsequent formation of a green phosphate/MO (V) complex at acidic pH17. Recent studies have shown that many flavonoids and related polyphenols contribute significantly to the antioxidant activity of many plants33.
Preliminary phytochemical analysis34 showed the presence of tannins and flavonoids in ethanolic extract of A. nilagirica. Thus the antioxidant potential of the plant extract could be attributed to the presence of polyphenolic compounds. In addition, the antioxidant activity may be due to enzymic and other non-enzymic antioxidants, which needs further analysis.
Acknowledgement
The authors express their sincere gratitude to the management of Karpagam University, Coimbatore.
References
- Govindarajan, R., Vijayakumar, M., Rawat, A.K.S. and Mehrotra, S., Free radical scavenging potential of Picrorhiza kurrooa Royle ex Benth, Indian J Exp Biol., 41, 875-879 (2003).
- Geesin, J.G., Gordon, J.S. and Berg, R.A., Retinoids affect collagen synthesis through inhibition of ascorbate-induced lipid peroxidation in cultured human dermal fibroblasts, Arch Biochem Biophys., 278, 352-356 (1990).
- Youdim, K.A. and Joseph, J.A., A possible emerging role of phytochemicals in improving age-related neurological dysfunctions-a multiplicity of effects, Free Rad Biol Med., 30, 583 (2001).
- Halliwell, B., Aeschbach, R., Loliger, J. and Aruoma O. I., The characterization of antioxidants, Food and Chemical Toxicology, 33, 601–617 (1995).
- Pratt, D.E., In: Phenolic compounds in Food and their effects on Health II: Antioxidants and Cancer prevention, by Mou-Tuan Huang, Chi-Tang Ho and Chang Lee (Eds), ACS Symposium Series 507, American Chemical Society, Washington, 54 (1992)
- Bhattacharjee, S., Hand Book of Medicinal Plants, Pointer Publishers, Jaipur. 43 (2000).
- Warrier, P.K., Nambiar, V.P.K. and Raman Kutty, C., Indian Medicinal Plants: a compendium of 500 species, Orient Longman Ltd; Chennai. 43 (1995).
- Yadav, A.K. and Temjenmongla, A., Anthelmintic Activity of Gynura angulosa DC. Against Trichinella spiralis infections in mice, Pharmacologyonline, 2, 299-306 (2006).
- Blois, M.S., Antioxidant determinations by the use of stable free radical, Nature,
- 81, 1199-2000 (1958).
- Oyaizu, M., Studies on products of browning reaction: antioxidant activities of products of browning reaction prepared from glucosamine, Jpn J Nut., 44, 307-315 (1986).
- Re, R. Pellegrini, N., Protoggenete, A., Pannala, A., Yang, M. and rice-Evans, C., Antioxidant activity applying an improved ABTS radical cation assay, Free Rad Biol Med., 26, 1231 (1999).
- Green, L.C., Wagner, D.A., Glogowski, J., Skipper, P.L., Wishnok, J.K. and Tannenbaum, S.R., Analysis of nitrate and 15N in biological fluids, Anal. Biochem., 126, 131-136 (1982).
- Marcocci, L., Maguire, J.J., Droy-Lefaix, M.T. and Packer, L., The nitric oxide scavenging property of Ginko biloba extract EGB 761, Biochem. Biophys. Res. Commun., 201, 748-755 (1994).
- Sanchez-Moreno C., Methods used to evaluate the free radical scavenging activity in foods and biological systems, Food Sci Tech Int., 8, 122 (2002).
- Kunchandy, E. and Rao, M.N.A., Oxygen radical scavenging activity of curcuminoid, Int J Pharmacognosy. 58, 237 (1990).
- Ohkawa, H., Ohishi, N. and Yagi, K., Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction, Anal Biochem., 95, 351 (1979).
- Prieto, P., Pineda, M. and Aguilar, M., Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application of vitamin E, Anal. Biochem., 269, 337-341 (1999).
- Singleton, V.L., Orthofer, R. and Lamuela-Raventos, R.M., Analysis of total phenols and oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent, Methods Enzymol., 299, 152-177 (1999).
- Zhishen, J., Mengcheng, T. and Jianming, W., The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food Chem., 64, 555-559 (1999).
- Cotran, R.S., Kumar, V. and Collins, T., In: Robbin’s pathological basis of disease, 6th edi., Thomson press (I) Ltd, Noida, India, 1 (1999).
- Sanchez Moreno, C., Larrauri, J. and Saura-Calixto, F., Free radical scavenging capacity of selected red and white wine, J Sci Food Agric, 791, 301 (1999).
- Rice-Evans, C., Miller, N.J., Factors affecting the antioxidant activity determined by the ABTS radical cation assay, Free Radic. Res., 195, 26-27 (1997).
- Lata, H. and Ahuja, G.K., Role of free radicals in health and disease, Ind J physio & Allied Sci, 57, 124 (2003).
- Ialenti, A., Moncada, S. and Di rosa, M., Modulation of adjuvant arthritis by endogenous nitric oxide, Br J Pharmacol, 110, 701 (1993).
- Ross, R., The pathogenesis of atherosclerosis: a perspective for the 1990’s, Nature, 362, 801 (1993).
- Sainani, G. S., Manika, J.S. and Sainani, R.G., Oxidative stress: a key factor in pathogenesis of chronic diseases, Med Update, 1, 1 (1997).
- Gutteridge, J.M.C., Age, pigments and free radicals: fluorescent lipid complexes by iron and copper containing proteins, Biochem Biophys Acta, 834, 144 (1978).
- Halliwell, B., Superoxide-dependent formation of hydroxyl free radicals in the presence of iron chelates, FEBS Lett., 92, 321 (1978).
- Braugghler, J.M., Duncan, C.A. and Chase, L.R., The involvement of iron in lipid
- peroxidation. Important of ferrous to ferric iron in initiation, J Biol Chem., 261, 10282 (1986).
- Meir, S., Kanner, J., Akiri, B. and Hadas, S.P., Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves, J Agric Food Chem., 43, 1813-1817 (1995).
- Hemmani, T. and Parihar, M.S., Reactive oxygen species and oxidative damage, Indian J Physiol Pharmacol., 42, 440 (1998).
- Khanamn, S. Shivaprasad, H.N. and Kshama, D., In vitro antioxidant screening models: a review, Indian J Pharm Educ., 38, 180 (2004).
- Luo, X.D., Basile, M.J. and Kennelly, E.J., Polyphenolic antioxidants from the fruits of Chrysophyllum cainito L. (star apple). J. Agric. Food Chem. 50, 1370-1382 (2002).
- Harborne, J.B., In: Phytochemical methods, Chapman and Hall Ltd, London, 356 (1987).

This work is licensed under a Creative Commons Attribution 4.0 International License.





