Manuscript accepted on : March 25, 2009
Published online on: 22-06-2009
V. Ravichandrian et al
VELS College of Pharmacy, Pallavaram, Chennai - 117 India.
ABSTRACT: Sustained release tablets of Ambroxol hydrochloride matrix tablets were developed using hydrophilic polymer HPMC K4M and Eudragit RSPO alone or combination with hydrophobic polymer Ethyl cellulose. The release study revealed that Eudragit RSPO or HPMC K4M was able to sustain the drug release up to 6hr and 8hr with ( 93.05±1.96, 96.12±1.74 ) respectively. While HPMC K4M or Eudragit RSPO with Ethyl cellulose sustained the drug release up to 12hrs with (95.64±1.02, 98.77±1.23). The in vitro release data was fit to Korsmeyer Peppas and the release mechanism was found to diffusion along with erosion and it is further confirmed by the SEM analysis. Hence the sustained release matrix tablet of Ambroxol hydrochloride attains therapeutic concentration in the blood plasma, leading to improved efficacy and better patient compliance.
KEYWORDS: Bronchitis; ambroxol hydrochloride; matrix tablets; HPMC K4M; Eudragit RSPO
Download this article as:| Copy the following to cite this article: Ravichandrian et al V. Formulation of Sustained Release Ambroxol Hydrochloride Matrix Tablets: Influence of Hydrophilic/Hydrophobic Polymers on The Release Rate and in Vitro Evaluation. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Ravichandrian et al V. Formulation of Sustained Release Ambroxol Hydrochloride Matrix Tablets: Influence of Hydrophilic/Hydrophobic Polymers on The Release Rate and in Vitro Evaluation. Biosci Biotechnol Res Asia 2008;6(1). Available from: https://www.biotech-asia.org/?p=8120 |
Introduction
Bronchitis is an inflammation of the bronchial tubes, or bronchi, that bring air into the lungs.1. Acute bronchitis usually lasts only a few days where as Chronic bronchitis lasts for many years. It is crucial for the success of bronchitis therapy to maintain the systemic drug concentration consistently throughout the course of treatment.2
Ambroxol hydrochloride, trans-4-[(2-amino,3,5-dibromobenzyl)amino cyclohexanol] group of (benzilamides) and is a metabolite of Bromhexine.It is an expectoration improver and mucolytic agent used in the treatment of acute and chronic bronchitis.. The limitation of ambroxol hydrochloride was its short biological half life, poor bioavailability and rapidly absorption from the gastrointestinal tract. The therapeutic effective plasma levels are slightly higher than 30ng/mL and can be achieved by 4 X 30 mg/day orally. The biological half life of Ambroxol hydrochloride is 3-4 hr, thus necessitating frequent administration to maintain constant therapeutic drug levels.3-5
As the drug is freely soluble at all pH, judicious selection of release-retarding excipients is necessary for achieving constant plasma concentration. The most commonly used method of modulating drug release is to include it in a matrix system.6 Introduction of matrix tablets as sustained released has given a new breakthrough for novel drug delivery system (NDDS) in the field of pharmaceutical technology. It excluded complex production procedures such as coating and pellitization during manufacturing and drug release rate from the dosage form is controlled by the type and proportion of polymer used in the preparations. Hydrophilic polymers form a gel like structure around the tablet core which controls the drug release. The hydrophilic polymers, HPMC K4 M or Eudragit RSPO selected in the present study provide pH-independent drug release to oral dosage forms that can be used for formulating the sustained-release dosage forms. However, the use of hydrophilic polymer alone for controlling the drug release of water soluble drugs is restricted due to rapid diffusion of the dissolved drug through the hydrophilic gel layer. Use of hydrophobic polymers will retard the drug release. So in the present investigation, an attempt has been made to formulate the controlled release matrix tablets of Ambroxol using hydrophilic matrix material (HPMC K4M or Eudragit RSPO) in combination with hydrophobic ethyl cellulose.7-12
Materials and Methods
Ambroxol hydrochloride was obtained as a gift sample from Milton Pharma Ltd., (Pondicherry,). HPMC K4 M, Eudragit RSPO and ethyl cellulose were purchased from Rankem limited (Mumbai). All other chemicals and reagents used in the study were of analytical grade and were used as procured.
Preparation of matrix tablets
Matrix tablets were prepared by direct compression technique using micro crystalline cellulose as directly compressible vehicle and magnesium stearate as lubricant. All the ingredients were sieved through 40 mesh screen and mixed with other ingredients and the powder mixture was compressed using single punch tablet machine. In total, 10 formulations containing different amounts of HPMC K4 M (F1, F2, F3), Eudragit RSPO (F4, F5, F6) and combination of HPMC K4 M or Eudragit RSPO with ethyl cellulose (F7, F8, F9, F10) were prepared and their formulae are presented in Table 1.
Characterization of blends
Prior to compression, blends were evaluated for their characteristic parameters like angle of repose,moisture content,carr’s index etc.The drug content in blends was determined by measuring the absorbance at 248 nm after suitable dilution.
Characterization of Tablets
The properties of the compressed matrix tablet, such as thickness hardness, friability, weight variation, ,content uniformity and drug content were determined using reported procedure.
In vitro release studies
The in vitro dissolution studies were performed using USP 22 dissolution apparatus No.2 (paddle) type at 50 rpm. The dissolution medium consists of 0.1 N hydrochloric acid for first 2 hr and for subsequent 10 hr in phosphate buffer pH 7.4 (900 ml), maintained at 37±0.5°C. The release studies were conducted in triplicate. Aliquots of samples (5 ml) were withdrawn at specific time intervals and drug content was determined spectrophotometerically at 248 nm. The in vitro release data obtained was treated to zero order rate equation, first order rate equation, Higuchi.s equation (Q = Kt1/2) and Korsmeyer-peppas to know precisely the mechanism of drug release from the matrix tablet
Results and Discussion
The blend for matrix tablet were prepared according to the formula given in Table 1 and characterized (Table 2). Angle of repose was less than 30° for all the batches of blends indicating satisfactory flow behavior. Other parameters for blends were also determined and found to be in acceptable range.
Table 1: Composition of sustained release matrix tablets of ambroxol hydrochloride
|
S.No |
BATCH |
F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 |
| (mg) | (mg) | (mg) | (mg) | (mg) | (mg) | (mg) | (mg) | (mg) |
(mg) |
||
| 1
|
Ambroxol
hydrochloride |
75 | 75 | 75 | 75 | 75 | 75 | 75 | 75 | 75 | 75 |
| 2 | HPMC | 20 | 40 | 60 | – | – | – | 30 | 20 | – | |
| 3 | Eudragit Rspo |
– | – | – | 20 | 40 | 60 | – | – | 30 | 20 |
| 4 | Ethyl Cellulose | – | – | – | – | – | – | 30 | 40 | 30 | 40 |
| 5
|
Magnesium
Stearate |
7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| 6 | Aerosil | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 7 | Microcrystalline cellulose | 195 | 175 | 155 | 195 | 175 | 155 | 155 | 155 | 155 | 155 |
| Total weight in mg | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | |
Table 2: Characterization of Blends
| Batch | Bulk density | True density | Carr’s index | Angle of repose |
| (g/ml) | (g/ml) | |||
| F1 | 0.63±0.012 | 0.78±0.014 | 19.2 | 280.0± 0.8 |
| F 2 | 0.53±0.015 | 0.65±0.012 | 18.5 | 250.2± 0.6 |
| F 3 | 0.39±0.012 | 0.53±0.016 | 20.4 | 270.8± 1.1 |
| F 4 | 0.48±0.013 | 0.61±0.015 | 21.3 | 280.2±0.6 |
| F 5 | 0.51±0.014 | 0.62±0.017 | 17.7 | 260.2±0.5 |
| F 6 | 0.38±0.015 | 0.54±0.015 | 19.5 | 240.1± 0.4 |
| F 7 | 0.46±0.013 | 0.63±0.016 | 22.9 | 260.2± 0.5 |
| F8 | 0.47±0.014 | 0.66±0.012 | 21.7 | 250.5± 0.7 |
| F9 | 0.38±0.014 | 0.54±0.014 | 29.6 | 280.2± 0.8 |
| F10 | 0.46±0.017 | 0.52±0.012 | 28.1 | 270.2± 0.8 |
Mean ± (s.d) (n=3)
Table 3: Characterization of Ambroxol hydrochloride matrix tablets.
| Batch | Thickness | Hardness | Friability | Average | Content |
| (mm) | (Kg/Cm2) | (%) | Weight | Uniformity | |
| (mg) | ( %) | ||||
| F1 | 3.87 | 6.7±0.5 | 0.24±0.002 | 298 ±2 | 98.3±2.4 |
| F 2 | 3.78 | 5.9±0.3 | 0.32±0.003 | 297±2 | 98.2±1.6 |
| F 3 | 3.56 | 6.2±0.5 | 0.38±0.005 | 301±4 | 98.7±2.2 |
| F 4 | 3.45 | 6.5±0.2 | 0.22±0.004 | 287±2 | 98.7±2.4 |
| F 5 | 3.98 | 7.1±0.3 | 0.11±0.007 | 295±4 | 98.6±1.3 |
| F 6 | 3.75 | 6.7±0.2 | 0.34±0.002 | 310±2 | 98.6±1.6 |
| F 7 | 3.23 | 6.2±0.2 | 0.36±0.003 | 305±3 | 99.4±1.2 |
| F8 | 3.84 | 5.9±0.4 | 0.33±0.004 | 288±3 | 98.8±1.8 |
| F9 | 3.93 | 6.0±0.2 | 0.25±0.003 | 295±3 | 99.2±1.4 |
| F10 | 3.83 | 6.2±0.8 | 0.32±0.003 | 298 ±2 | 98.7±2.2 |
Mean ± (s.d) (n=3)
The tablets of different formulations were subjected to various evaluation tests.Good uniformity in drug content was found among different batches of the tablets, and the drug content was more than 95% (Table 2).
In-vitro dissolution studies
Fig. 1 shows the effect of different concentrations of HPMC K4 M 10%(F1), 15% (F2) and 20% wt/wt of tablet (F3) on% release (98.61%, 98.11% and 96.12% within 5 hr, 6 hr and 8 hr of dissolution study, respectively) of Ambroxol. No significant difference in release rate was observed between tablets containing either 10% or 15% of HPMC K4M. Drug release was decreased significantly when 20% of HPMC K4 M was used in formulation F3. However, 30-40% of drug was released in first 2.5 hr of dissolution. In the in vitro study of matrix tablets containing 10%, 15% and 20% of Eudragit RSPO (F4, F5 and F6), it was observed that tablets containing 10% and 15% were eroded within 1h of the dissolution study indicating insufficient quantity of Eudragit RSPO form the gelatinous layer around the tablet core. Tablets containing 20% of Eudragit RSPO were able to form the gelatinous layer around the tablet core and the drug release was found to be 93.05% within 6 hr of dissolution study. However 40% – 60% of drug was released in the first 2.5 hr of dissolution study (Fig. 3)
 |
Figure 1: Dissolution profile of F1, F2 and F3.
|
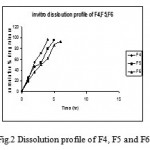 |
Figure 2: Dissolution profile of F4, F5 and F6.
|
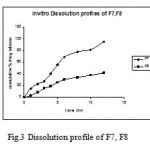 |
Figure 3: Dissolution profile of F7, F8 .
|
Therefore, in the next batch of tablets, to control the initial burst release ethyl cellulose was included in the matrix in the ratio of 1:1 and 1:2 along with HPMC K4M (F7, F8) or Eudragit RSPO (F9, F10 ) which resulted in extending the drug release for a period of 12h for (F7, F9 ) indicating fair uniform drug release through out the dissolution period (fig. 3). This may be due to a more rigid complex formed by hydrophilic polymers (HPMC K4 M and Eudragit RSPO) in presence of ethyl cellulose, which helped in retaining the drug in the matrix and did not allow rapid diffusion of soluble drug from the matrix. The tablets formulated (F7, F9 ) using combination of HPMC K4 M or Eudragit RSPO and ethyl cellulose did not show any burst release The hydrophobic nature of ethyl cellulose seems to have contributed toward reduction in the penetration of the solvent molecules into the matrix.
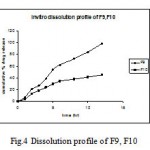 |
Figure 4: Dissolution profile of F9, F10.
|
Increasing the concentration of ethyl cellulose shows more retardation in the release of the drug from the formulation (F8) and (F10) containing ethyl cellulose in combination with HPMC K4M and Eudragit RSPO in the ratio 1:2, respectively. F8 showed only 41.4% and F10 showed 45.21% of drug release in 12 h of dissolution study which may result in insufficient therapeutic concentration. Considering the decrease in the therapeutic effect on lowering the concentration of hydrophilic polymers over the dissolution release, the effect of only ethyl cellulose was not carried out.
To know the kinetics of drug release, the data was treated according to different models. Best in vitro released formulations (F7, F9) were only studied for kinetics of drug release and showed best fir into Korsmeyer equation (0.991, 0.9775, respectively) and indicated combined effect of diffusion with erosion mechanisms of drug release.
SEM study further confirmed both diffusion and erosion mechanisms to be operative during drug release from the optimized batch of matrix tablet (F7 only). SEM photomicrograph of the matrix tablet taken at different time intervals after the Dissolution experiment showed that matrix was intact and pores had formed throughout the matrix (fig no.5). SEM photomicrographs of tablet surface at different time intervals also showed that erosion of matrix increased with respect to time indicated by the photomicrographs at 2, 5 and 10 h revealing pores with increasing diameter. These photomicrograph also revealed the formation of gelling structure indicating the possibility of swelling of matrix tablets (fig no. 6 and 7). Hence, the formation of both pores and gelling structure on tablet surface indicates the involvement of both erosion and diffusion mechanisms to be responsible for sustaining the release of Ambroxol Hcl from formulated matrix tablets.
 |
Figure 5: after 2 hr.
|
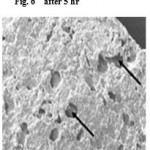 |
Figure 6: after 5 hr.
|
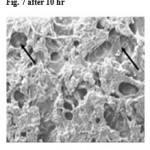 |
Figure 7: after 10 hr.
|
Overall it can be concluded that, the presence of ethyl cellulose as well as the % of total matrix material significantly influenced the release rate of drug. The tablets with 20% of matrix material (1:1) ratio of HPMC K4 M or Eudragit RSPO and ethyl cellulose gave satisfactory results on the formulation of controlled release dosage form of Ambroxol hydrochloride.
Conclusion
Accordingly, major object of the present invention is the provision of improved sustained released ambroxol hydrochloride dosage form that slowly release its active ingredients, such that it will maintain therapeutically effective levels of the active drug in the blood stream for a prolonged period of time. Results of present study demonstrated that combination of both hydrophilic and hydrophobic polymers could be successfully employed for formulating sustained release matrix tablets of ambroxol hydrochloride. The investigated sustained release matrix tablet was capable of maintaining constant plasma concentration through 12 hours. This can be expected to reduce the frequency of administration, side effects associated with repeated administration of conventional ambroxol hydrochloride tablets.
References
- Goodman Gilman, The pharmacological basis of therapeutics. In Hardman J, Limbid LE, editors Mc Graw- Hill, 10th 1024, 1990.
- Roger Walker, Cate Whittlesea, Clinical Pharmacy and therapeutics. Churchill Living stone 4th 386 2006
- United States Pharmacopoeia, 25th, The United States Pharmacopoeial convention, Inc., Rockville, 2002, pp. 1821-1824.
- Wataru Kubo, Shozo Miyazaki, Masatake, Mitsuo Togashi, Ryozo Mikami, David Attwood,2004,Oral sustained delivery of ambroxol from in situ-gelling pectin formulations, International Journal of Pharmaceutics,271,233-240.
- Wen-Ting Ke, Tien-Tzu, Hsiu-O, physical and chemical characterization of Ambroxol SR matrix tablets containing melt coated granules of Ambroxol with compritol 888, Asian of pharmaceutical sciences, 1, 35-42, 2006.
- Mehsali MM, EL-Syed. Preparation and evaluation of Theophyllin sustained release tablets. Drug develop Ind. Pharm, 373-376, 1996.
- Raghuram Reddy, Srinivas Mutalik, and Srinivas Reddy, Once-Daily Sustained-Release Matrix Tablets of Nicorandil: Formulation and In Vitro Evaluation, AAPS PharmSciTech; 4 (4) Article 61, pp1-8, 2003.
- C.Gohel, R.K.parikh, M.N.padshala, Formulation of directly compressible Isoniazid modified release matrix tablet, Indian journal of pharmaceutical sciences, pp 640-645, 2007
- R. Bhalekar, A. R. Madgulkar, D. D. Sheladiya, S. J. Kshirsagar, N. D. Wable S. S. Desale, Statistical Optimization of Sustained Release Venlafaxine HCl Wax Matrix Tablet, Indian journal of pharmaceutical sciences, pp 472-476, 2008.’
- Saleh M. Al-Saidan,1 Yellela S.R. Krishnaiah,1 Srinivas S. Patro,2 and Vemulapalli Satyanaryana, In Vitro and In Vivo Evaluation of Guar Gum Matrix Tablets for OralControlled Release of Water-soluble Diltiazem Hydrochloride, AAPS PharmSciTech; 6 (1) Article 5 pp E14-E21,
- Xue-mei Feng,Qi Ren,Wen-zhi Zhang,Hui-feng Shen,Zheng-xing Rong,Chao Fang and Hong-zhuan Chen,2008,Preparation and evaluation of a novel delayed-onset sustained release system of propranolol hydrochloride., Journal of pharmacy and pharmacology,60,817-822.
- Hosseinali Tabandeh,Seyed Alireza Mortazavi,Bassir Guilani 2003,Preparation of sustained released matrix tablets of asprin with ethyl cellulose,eudragit RS 100 and Eudragit S100 and studying the release profiles and their sensitivity to tablet hardness.,Iranian journal of pharmaceutical research,201-206.

This work is licensed under a Creative Commons Attribution 4.0 International License.





